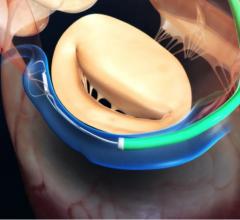
January 13, 2021 — FineHeart, a preclinical-stage medical device company developing a novel left ventricular assist ...
Heart Failure
This heart failure channel offers news and new technology to treat heart failure. This includes for new innovations to treat congestive heart failure (CHF). The channel includes news on HFpEF and HFrEF. Heart failure occurs when the heart is no longer able to pump as much blood as the body requires. This can lead to enlargement of the heart because the muscle works harder to supply blood, but the pumping is ineffective. This may be due to defects in the myocardium, such as an infarct, or due to structural issues such as severe valve regurgitation. The disease is divided into four New York Heart Association (NYHA) classes. Stage IV heart failure is when the heart is completely failing and requires a heart transplant or a left ventricular assist device (LVAD).
December 29, 2020 — Here is a list of the top 10 news stories from the European Society of Cardiology (ESC) in 2020 that ...
December 21, 2020 — U.S. Food and Drug Administration (FDA) approved updated labeling December 17 for Abbott's HeartMate ...
New research shows superiority of Ultromics’ AI in predicting cardiac-related death As the use of artificial ...
December 2, 2020 — A 2-month-old infant diagnosed with COVID-19 (SARS-CoV-2) experienced reversible myocardial injury ...
December 2, 2020 — The Centers for Medicare and Medicaid Services (CMS) finalized updates to Medicare coverage policies ...
November 18, 2020 — A late-breaking study at the 2020 American Heart Association (AHA) Scientific Sessions showed nearly ...
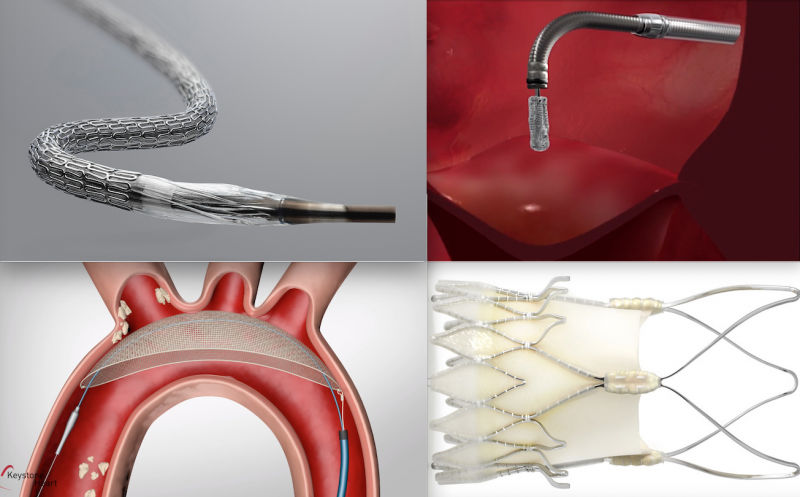
Here are some of the key takeaways from the late-breaking interventional cardiology and structural heart trials ...
The late-breaking MitraBridge Study was presented at Transcatheter Cardiovascular Therapeutics (TCT) 2020 meeting showed ...
October 14, 2020 — American College of Cardiology (ACC) Quality Summit virtual meeting Oct. 8-9, 2020, featured several ...

This is an overview of some of the biggest cardiology technology advances. These innovations are covered in more detail ...
October 5, 2020 — The American College of Cardiology (ACC) says heart failure (HF) is a cardiovascular epidemic. In ...
October 2, 2020 — Cardiac Dimensions announced the company has closed a $17.5 million Series C financing that will be ...
September 23, 2020 – A team of cardiologists at Allegheny General Hospital (AGH), part of Allegheny Health Network (AHN) ...
September 8, 2020 - Heart patients hospitalized with COVID-19 (SARS-CoV-2) can safely continue taking angiotensin ...
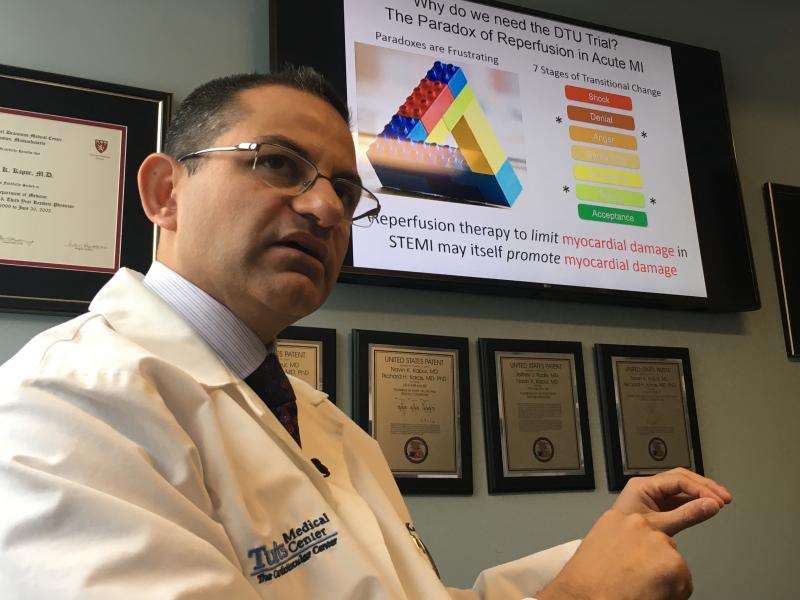
The cardiology program at Tufts Medical Center in Boston is internationally recognized for being on the forefront of ...


 January 13, 2021
January 13, 2021