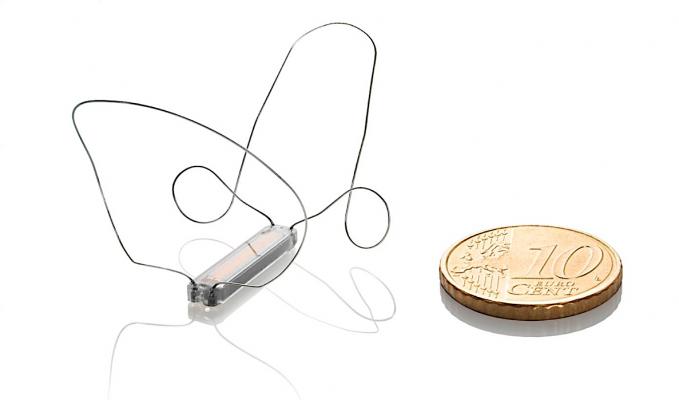
November 21, 2019 — Endotronix, a digital health and medical technology company dedicated to advancing the treatment of chronic heart failure (HF), presented positive first-in-human data of the Cordella Pulmonary Artery (PA) Pressure Sensor System. Data was presented at the 2019 American Heart Association (AHA) scientific sessions by Prof. Dr. Wilfried Mullens of the Hospital Oost-Limburg in Genk, Belgium.
"Chronic heart failure patients too frequently have cycles of decompensation and hospitalization that negatively impact patient outcomes and drive up treatment costs. Recent data shows that using PA pressure-guided therapy with this population keeps them healthier and out of the hospital," Mullens explained. "The Cordella System is an elegant solution that integrates a PA pressure sensor and a comprehensive patient management platform in one. The straightforward sensor implant provides reliable pressure data, while the patient management platform enables a comprehensive clinical picture of the patient that allows my team to make informed therapy decisions and improve outcomes."
The 90-day results from the first-in-human trial confirm the device safety and accuracy of pressure measurements in the right pulmonary artery using the implanted Cordella Sensor and handheld patient reader. The study included 15 patients across two European sites: Mullens in Belgium and Dr. Faisal Sharif of the National University of Ireland (NUI) in Galway, Ireland.
SIRONA Trial Highlights
The primary accuracy endpoint was met with no significant difference in measured mean PA pressure for the Cordella Sensor and the reference catheter at 90 days.
The study demonstrated encouraging safety results with zero device related system complications (DSRC) at 90 days.
High patient compliance (>98%) with daily remote measurements of vital signs and PA pressure throughout the study.
"The presentation of our first-in-human data at AHA this year is another exciting milestone for the company," said Dr. Katrin Leadley, Chief Medical Officer of Endotronix. "This work lays the clinical foundation for our groundbreaking IDE trial, PROACTIVE-HF, which will begin enrollment in the U.S. later this year. Designed to provide the highest level of clinical evidence for PA pressure-guided therapy, PROACTIVE-HF will support U.S. market access of the Cordella Sensor and inform a national coverage decision from the Centers for Medicare & Medicaid Services (CMS)."
The Cordella Sensor is not available for commercial use in any geography and is under clinical investigation in Europe (SIRONA II CE Mark Trial) and the U.S. (PROACTIVE-HF IDE Trial).
The Cordella Heart Failure System
The Cordella system is designed to help patients suffering from chronic heart failure feel better and stay out of the hospital with streamlined care and remote medication titration. The system provides a comprehensive health status of the patient at home with easy-to-use tools to securely collect and share health related data with healthcare providers for trend-based management. The Cordella Sensor seamlessly integrates pulmonary artery (PA) pressure data into the Cordella System. Together, they proactively deliver the information necessary to improve patient care between office visits and support reimbursement for care delivery activities.
For more information: www.endotronix.com
Find links to all the AHA late-breaking trials and other key news.


 November 14, 2025
November 14, 2025