November 19, 2021 — Adults treated with empagliflozin during hospitalization for acute heart failure experienced a ...
Heart Failure
This heart failure channel offers news and new technology to treat heart failure. This includes for new innovations to treat congestive heart failure (CHF). The channel includes news on HFpEF and HFrEF. Heart failure occurs when the heart is no longer able to pump as much blood as the body requires. This can lead to enlargement of the heart because the muscle works harder to supply blood, but the pumping is ineffective. This may be due to defects in the myocardium, such as an infarct, or due to structural issues such as severe valve regurgitation. The disease is divided into four New York Heart Association (NYHA) classes. Stage IV heart failure is when the heart is completely failing and requires a heart transplant or a left ventricular assist device (LVAD).
November 19, 2021 — Stem cell therapy helped to reduce the number of heart attacks, strokes and death among people with ...
November 17, 2021 — Canagliflozin, a medication used to treat type 2 diabetes, was found to greatly improve symptoms and ...
New research shows superiority of Ultromics’ AI in predicting cardiac-related death As the use of artificial ...
November 10, 2021 — Ancora Heart Inc., a company developing a novel therapy to address heart failure, announced results ...
November 2, 2021 — Cardionomic Inc. announce initial U.S. enrollment in its global Cardiac Pulmonary Nerve Stimulation ...

November 1, 2021 — Here is the list of the most popular content on the Diagnostic and Interventional Cardiology (DAIC) ...
October 27, 2021 — Impulse Dynamics announced the U.S. Food and Drug Administration approved a modification of labeling ...
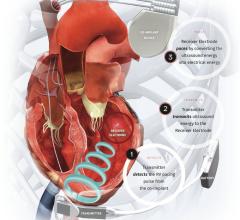
October 27, 2021 — EBR Systems Inc., developer of the world’s first wireless cardiac pacing system for heart failure, ...
October 20, 2021 – CorWave, a French medtech company developing a next-generation heart pump, won the 2021 HealthTech ...
October 7, 2021 – The U.S. Food and Drug Administration (FDA) recently granted the breakthrough therapy designation for ...
October 4, 2021 – Nanowear, a hospital-at-home and remote diagnostic platform that used proprietary wearable cloth ...
Mechanical engineering Professor Nathan Sniadecki, associate chair for research and infrastructure, mechanical ...
October 1, 2021 — A pooled analysis of two randomized trials has demonstrated the beneficial effects of empagliflozin in ...
September 28, 2021 — Hemodynamic guided management using the CardioMEMS device may reduce heart failure hospitalizations ...
September 22, 2021 — A cardiothoracic surgical team with University of Louisville Health – Jewish Hospital has performed ...


 November 19, 2021
November 19, 2021




![Figures from an initial study on the Cardionomic CPNS technology in a poster presentation at the Heart Rhythm Society (HRS) 2021 meeting.[1] Starting at top left, an angiographic view of the atrial transseptal access for a left ventricular septal ablation. Procedural intra-cardiac echo (ICE) showing ablation catheter positioning. A 3D electro-anatomic map of the LV septum. The final graphs show baseline and post-procedure LVOT pressure readings demonstrating a decreased gradient.](/sites/default/files/styles/content_feed_medium/public/Cardionomic_Cardiac_Pulmonary_Nerve_Stimulation_CPNS_for_HF.jpg?itok=ecrU9hmQ)