It is estimated that more than 10 million people in the United States are affected by peripheral arterial disease (PAD) ...
Guidewires
This channel includes news and new technology innovations for guide wires used in interventional cardiology and interventional radiology. Guidewires are used to navigate vessels to target lesions so larger, less maneuverable treatment devices can be pushed over the wire to rapidly reach the lesion.
February 19, 2016 — Nipro North America announced the launch of the AquaLiner II second-generation 0.035-inch ...
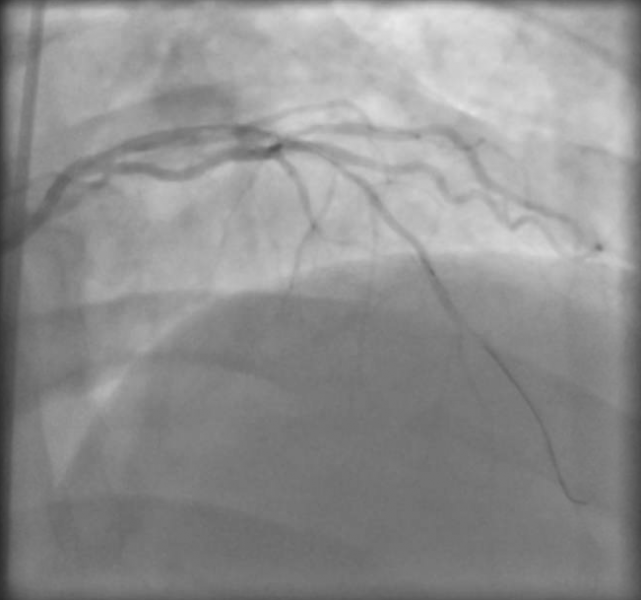
November 24, 2015 — The U.S. Food and Drug Administration (FDA) issued a warning this week that hydrophilic and/or ...

March 4, 2014 — The U.S. Food and Drug Administration (FDA) has sent out a Class 1 recall notice regarding various ...
January 24, 2014 — The U.S. Food and Drug Administration (FDA) cleared Radius Medical LLC’s Prodigy Support Catheter ...
October 30, 2013 — Terumo Interventional Systems announced the launch of the 0.018-inch Glidewire Advantage peripheral g ...
July 30, 2013 — Biotronik has launched its next generation of guidewires. Galeo Pro has a high tensile stainless steel ...
July 18, 2013 — Boston Scientific Corp. has received U.S. Food and Drug Administration (FDA) 510(k) clearance and CE ...
May 15, 2013 — Vascular Solutions Inc. announced that it has re-launched the Venture catheter, a deflectable-tip cathete ...

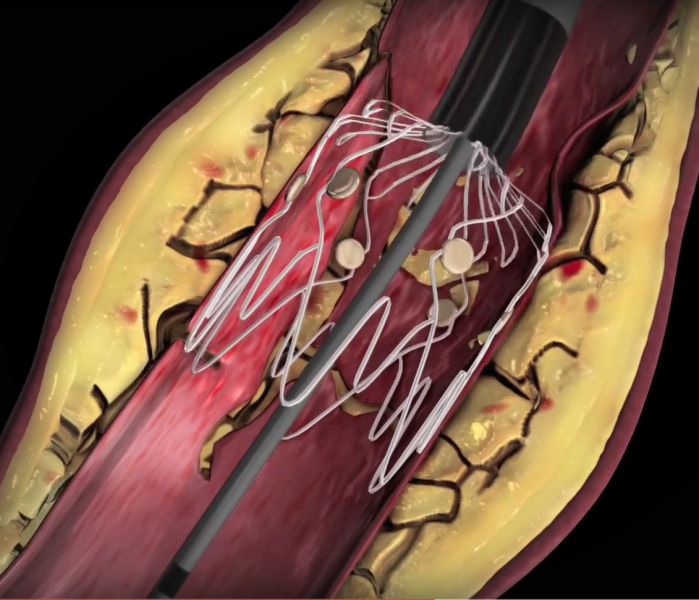
 February 22, 2016
February 22, 2016





