May 19, 2014 — St. Jude Medical announced that data presented during a late-breaking clinical trial session at the 2014 ...
EP Lab
This channel includes news and new technology innovations for cardiac electrophysiology (EP) systems, techniques and devices using in EP labs. This includes implantable EP devices, pacemakers, implantable cardioverter defibrillators (ICD), cardiac resychronization therapy (CRT), ablation technologies, left atrial appendage (LAA) occlusion, atrial fibrilation (AF) and Holter monitors.

May 16, 2014 — New data presented as a late-breaking clinical trial at the Heart Rhythm Society's (HRS) 2014 annual ...

May 14, 2014 — Data presented during Heart Rhythm 2014 found the use of quadripolar leads reduced the number of ...
When the patients of Michael Boler, M.D. need cardiac monitoring, the Holter monitor is no longer his first choice. “The ...

May 14, 2014 — Vivek Reddy, M.D., director of arrhythmia services for The Mount Sinai Hospital, reported promising 12 ...

May 13, 2014 — The Heart Rhythm Society (HRS), American College of Cardiology (ACC) and American Heart Association (AHA) ...

May 13, 2014 — A Boston Scientific Corp. study demonstrated that outcomes for patients with the company's transvenous im ...
Apple created a stir when it announced in 2018 that its Apple Watch Series 4 was the first consumer health and fitness ...
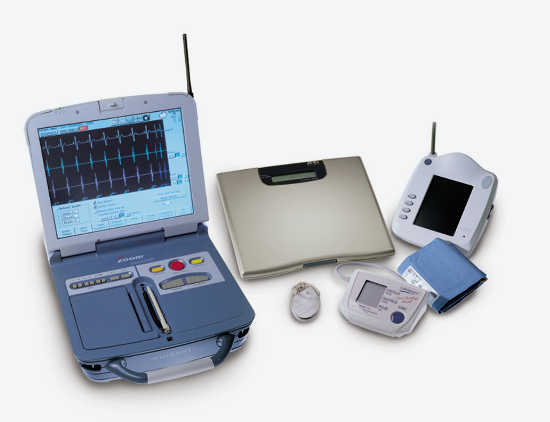
May 12, 2014 — Patients using the Boston Scientific Latitude remote patient management system with wireless telemetry ...
May 12, 2014 — Kalila Medical received 510(k) clearance from the U.S. Food and Drug Administration (FDA) for its Vado ...
May 9, 2014 — A quarter century ago, doctors treating patients with implanted cardiac pacemakers had a big problem ...
Washington Health System (WHS) provides healthcare services at more than 40 offsite locations across three counties in ...
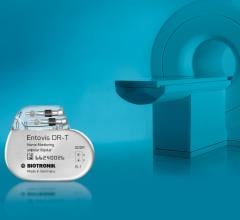
May 8, 2014 — The Food and Drug Administration (FDA) has granted Biotronik approval for its Entovis pacemaker system ...
May 7, 2014 — Cardiotek B.V., a subsidiary of Schwarzer, a privately held medical device company focused on electrophysi ...
May 6, 2014 — Medtronic announced the first U.S. implant of the Evera MRI SureScan implantable cardioverter ...
Apple created a stir when it announced in 2018 that its Apple Watch Series 4 was the first consumer health and fitness ...

The American College of Cardiology (ACC) annual meeting is a key event where vendors launch new cardiology technologies ...
May 6, 2014 — CardioInsight Technologies Inc.’s Ecvue system will be featured at the Rhythm Theatre Symposium and in 17 ...
April 30, 2014 — Scripps Health became the first health care provider in Southern California to implant the ...

 May 19, 2014
May 19, 2014