Here is the list of the most popular articles and videos on the Diagnostic and Interventional Cardiology (DAIC) magazine ...
EP Lab
This channel includes news and new technology innovations for cardiac electrophysiology (EP) systems, techniques and devices using in EP labs. This includes implantable EP devices, pacemakers, implantable cardioverter defibrillators (ICD), cardiac resychronization therapy (CRT), ablation technologies, left atrial appendage (LAA) occlusion, atrial fibrilation (AF) and Holter monitors.
November 25, 2017 — Sitting in, or standing close to the charging port of a Tesla electric vehicle did not trigger a ...
November 16, 2017 — Bristol-Myers Squibb Company and Pfizer Inc. released real-world data (RWD) of outcomes associated ...
When the patients of Michael Boler, M.D. need cardiac monitoring, the Holter monitor is no longer his first choice. “The ...
Vivek Reddy, M.D., director of cardiac arrhythmia services and professor of medicine, cardiology, Mount Sinai Hospital ...
November 10, 2017 — Mexican doctors have safely reused donated pacemakers after sterilization, according to a study ...
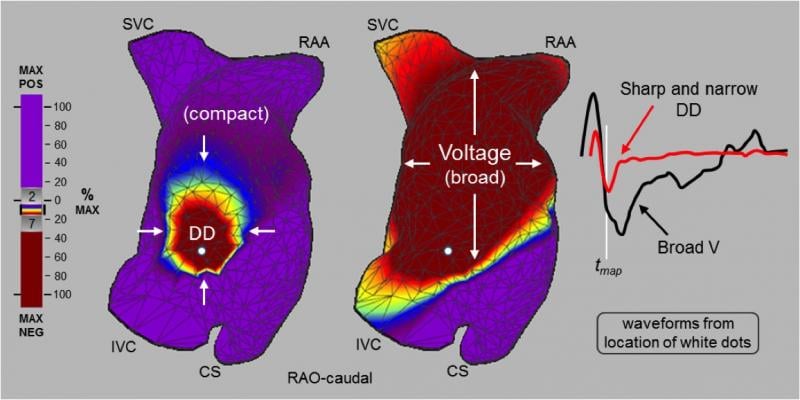
November 7, 2017 — Here is an aggregated list of articles detailing the latest clinical data and new device technology ...
Apple created a stir when it announced in 2018 that its Apple Watch Series 4 was the first consumer health and fitness ...

November 3, 2017 — Here is the list of the most popular articles and videos on the Diagnostic and Interventional ...

November 2, 2017 – Five-year results from the PREVAIL Trial comparing left atrial appendage closure (LAAC) with the ...
October 30, 2017 — The American College of Cardiology, along with the American Heart Association and the Heart Rhythm ...
Washington Health System (WHS) provides healthcare services at more than 40 offsite locations across three counties in ...
This video, provided by Acutus Medical, demonstrates a patient case showing the use of the AcQMap high-resolution ...
October 24, 2017 — Acutus Medical announced that the U.S. Food and Drug Administration (FDA) has cleared the AcQMap High ...
October 23, 2017 — University of North Carolina (UNC) School of Medicine cardiologist Anil Gehi, M.D., will use a $1.7 ...
Apple created a stir when it announced in 2018 that its Apple Watch Series 4 was the first consumer health and fitness ...
October 23, 2017 – The U.S. Food and Drug Administration (FDA) has cleared Abbott’s Confirm Rx Insertable Cardiac ...
October 20, 2017 — Boston Scientific announced new data from the Multisensor Chronic Evaluation in Ambulatory Heart ...
October 19, 2017 — Novel smartphone and tablet applications for atrial fibrillation patients and healthcare ...


 December 06, 2017
December 06, 2017