May 15, 2018 — According to new research, smoking marijuana may not be associated with an increased risk of ventricular ...
EP Lab
This channel includes news and new technology innovations for cardiac electrophysiology (EP) systems, techniques and devices using in EP labs. This includes implantable EP devices, pacemakers, implantable cardioverter defibrillators (ICD), cardiac resychronization therapy (CRT), ablation technologies, left atrial appendage (LAA) occlusion, atrial fibrilation (AF) and Holter monitors.
May 15, 2018 – Results of the AVIATOR 2 international registry data show a discrepancy between physician perception and ...
May 11, 2018 — A new study finds that integrating two separate clinical risk score models more accurately helps ...
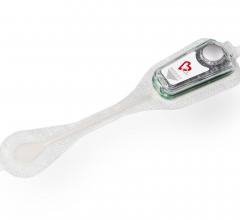
When the patients of Michael Boler, M.D. need cardiac monitoring, the Holter monitor is no longer his first choice. “The ...
May 10, 2018 — Cardiva Medical Inc. announced the completion of the AMBULATE pivotal trial, one of several clinical ...
May 10, 2018 — Results from the clinical study to evaluate Imricor’s Vision-MR Ablation Catheter for the treatment of ...
Apple created a stir when it announced in 2018 that its Apple Watch Series 4 was the first consumer health and fitness ...
May 9, 2018 — The U.S. Food and Drug Administration (FDA) said it recently granted market clearance for Angel Medical ...
A new clinical trial at The Ohio State University Wexner Medical Center is examining an implanted device that uses vagus ...
May 9, 2018 — Stroke rehabilitation specialists at the The Ohio State University Wexner Medical Center are among the ...
Washington Health System (WHS) provides healthcare services at more than 40 offsite locations across three counties in ...
May 8, 2018 — Biosense Webster Inc. announced its Carto Vizigo Bi-directional Guiding Sheath is now available in the ...
May 8, 2018 — Bardy Diagnostics Inc. (BardyDx) announced that the American Heart Journal has published the results of a ...

May 7, 2018 — The U.S. Food and Drug Administration (FDA) has approved Portola Pharmaceuticals' Andexxa, the first ...
Apple created a stir when it announced in 2018 that its Apple Watch Series 4 was the first consumer health and fitness ...
May 4, 2018 — Stereotaxis and Acutus Medical announced a strategic collaboration to integrate the Stereotaxis Niobe ...
May 3, 2018 — APN Health LLC announced its Navik 3D mapping system is commercially released and in clinical use. The ...
May 3, 2018 — The U.S. Food and Drug Administration (FDA) has granted market clearance for the Abbott Advisor HD Grid ...


 May 15, 2018
May 15, 2018