December 20, 2018 — Cardiva Medical Inc. announced the company has received premarket approval (PMA) from the U.S. Food ...
EP Lab
This channel includes news and new technology innovations for cardiac electrophysiology (EP) systems, techniques and devices using in EP labs. This includes implantable EP devices, pacemakers, implantable cardioverter defibrillators (ICD), cardiac resychronization therapy (CRT), ablation technologies, left atrial appendage (LAA) occlusion, atrial fibrilation (AF) and Holter monitors.
December 18, 2018 — Irish medical device company AuriGen Medical won the prestigious Global Innovation Award at the ...
December 14, 2018 — The Department of Health and Human Services (HHS) recently entered into an interagency agreement ...
When the patients of Michael Boler, M.D. need cardiac monitoring, the Holter monitor is no longer his first choice. “The ...
December 10, 2018 — Electrophysiology, while currently a relatively small market at $4.6 billion in 2018, has recently ...

December 5, 2018 — Supraventricular tachycardia (SVT) is a common heart abnormality that presents as a fast heart rate ...
November 30, 2018 — Johnson & Johnson Medical Devices Companies announced that Biosense Webster Inc. has enrolled and ...
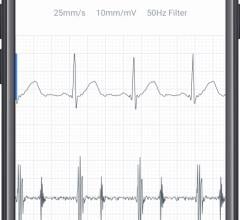
Apple created a stir when it announced in 2018 that its Apple Watch Series 4 was the first consumer health and fitness ...
DAIC Editor Dave Fornell takes a tour of some of the most innovative new cardiovascular technologies on display on the ...
Wilber Su, M.D., chief of cardiac electrophysiology, Banner University Medical Center, Phoenix, and clinical associate ...
November 12, 2018 — The Vascade MVP vascular closure system met its endpoints compared to manual compression in a ...
Washington Health System (WHS) provides healthcare services at more than 40 offsite locations across three counties in ...
November 12, 2018 — At the American Heart Association’s (AHA) Scientific Sessions 2018, Nov. 10-12 in Chicago, Eko ...
November 9, 2018 — The American College of Cardiology (ACC), the American Heart Association (AHA) and the Heart Rhythm ...

November 7, 2018 — Here is a list of some of the key clinical trial presentations at the 2018 American Heart Association ...
Apple created a stir when it announced in 2018 that its Apple Watch Series 4 was the first consumer health and fitness ...
October 29, 2018 — Good Samaritan Hospital recently became the first hospital on the West Coast to utilize the Carto ...

October 17, 2018 — The U.S. Food and Drug Administration (FDA) has reviewed information about potential cybersecurity ...

Sudden cardiac death (SCD) is the leading medical cause of death in young athletes and its impact is consistent ...


 December 20, 2018
December 20, 2018