October 7, 2019 — Glynn Crawford was hospitalized at University Hospitals Ahuja Medical Center in late June/early July ...
EP Lab
This channel includes news and new technology innovations for cardiac electrophysiology (EP) systems, techniques and devices using in EP labs. This includes implantable EP devices, pacemakers, implantable cardioverter defibrillators (ICD), cardiac resychronization therapy (CRT), ablation technologies, left atrial appendage (LAA) occlusion, atrial fibrilation (AF) and Holter monitors.

Clifford Robinson, M.D., associate professor of radiation oncology, chief of the SBRT service, director of clinical ...
September 20, 2019 — BioTrace Medical Inc. announced the company’s key activities at the 31st annual Transcatheter ...
When the patients of Michael Boler, M.D. need cardiac monitoring, the Holter monitor is no longer his first choice. “The ...
September 20, 2019 — The new Max and Debra Ernst Heart Center at Beaumont Hospital, Royal Oak, Mich., will open Sept. 30 ...
September 18, 2019 — Early use of an implantable cardioverter-defibrillator (ICD) after primary coronary intervention ...
September 17, 2019 — Treating high-risk heart patients with a single, high dose of radiation therapy can dramatically ...
Apple created a stir when it announced in 2018 that its Apple Watch Series 4 was the first consumer health and fitness ...

Atrial fibrillation, or Afib, kills about 130,000 people worldwide every year. It also affects 3 to 6 million ...
August 27, 2019 – Lenox Hill Hospital (New York, N.Y.) has established a brand new Heart Rhythm Center dedicated to the ...
Washington Health System (WHS) provides healthcare services at more than 40 offsite locations across three counties in ...
August 19, 2019 — The U.S. Food and Drug Administration (FDA) granted market clearance the Barostim Neo System for the ...
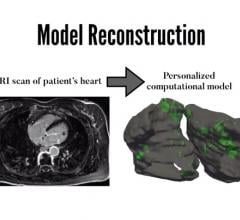
August 19, 2019 — Scientists at Johns Hopkins have successfully created personalized digital replicas of the upper ...
August 15, 2019 — Thin, flexible fibers made of carbon nanotubes have now proven able to bridge damaged heart tissues ...
Apple created a stir when it announced in 2018 that its Apple Watch Series 4 was the first consumer health and fitness ...
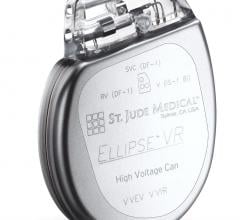
August 5, 2019 — Abbott is recalling the Ellipse Implantable Cardioverter Defibrillators (ICDs) because electrical ...
July 31, 2019 — The chances of patients experiencing complications after having a cardiac device implanted vary ...
Mark Ibrahim, M.D., FACC, assistant professor of medicine and radiology, associate program director, advanced cardiac ...

 October 07, 2019
October 07, 2019