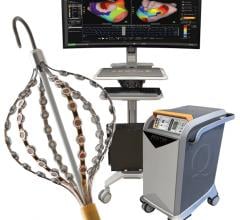
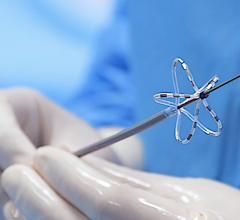
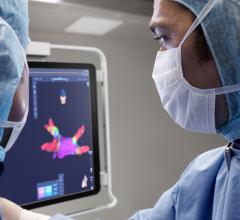
October 8, 2020 — Galaxy Medical, a developer of pulsed electric field (PEF) technology for the treatment of cardiac ...
EP Lab
This channel includes news and new technology innovations for cardiac electrophysiology (EP) systems, techniques and devices using in EP labs. This includes implantable EP devices, pacemakers, implantable cardioverter defibrillators (ICD), cardiac resychronization therapy (CRT), ablation technologies, left atrial appendage (LAA) occlusion, atrial fibrilation (AF) and Holter monitors.
October 8, 2020 – People who suffer from persistent atrial fibrillation in the heart may find relief from a new ...
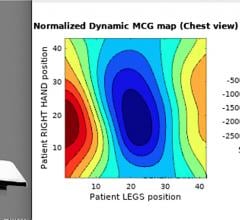
October 6, 2020 — A new technology being developed by U.S.-based Mesuron Inc. using a superconducting quantum ...
When the patients of Michael Boler, M.D. need cardiac monitoring, the Holter monitor is no longer his first choice. “The ...
October 6, 2020 – The U.S. Food and Drug Administration (FDA) cleared the Biosense Webster ThermoCool SmartTouch SF ...
Horst Sievert, M.D., is the director of the Cardiovascular Center Frankfurt, and associate professor of internal ...
October 1, 2020 — The U.S. Food and Drug Administration (FDA) granted 510(k) clearance for the SentiAR CommandEP system ...
Apple created a stir when it announced in 2018 that its Apple Watch Series 4 was the first consumer health and fitness ...
Peter Weiss M.D., MSc, director of ventricular arrhythmia management and robotics, and assistant clinical professor of ...
Peter Weiss M.D., MSc, director of ventricular arrhythmia management and robotics, and assistant clinical professor of ...
September 29, 2020 — The U.S. Food and Drug Administration (FDA) granted 510(k) clearance for the second-generation of ...
Washington Health System (WHS) provides healthcare services at more than 40 offsite locations across three counties in ...
September 25, 2020 — Short-term hydroxychloroquine treatment is not associated with lethal heart rhythms in patients ...
September 25, 2020 — Remote and in-hospital wearable biosensor technology company VitalConnect Inc. has started the ...
September 24, 2020 — Fitbit received 510(k) clearance from the U.S. Food and Drug Administration (FDA), as well as ...
Apple created a stir when it announced in 2018 that its Apple Watch Series 4 was the first consumer health and fitness ...
September 23, 2020 — Boston Scientific Corp. signed an investment agreement with an exclusive option to acquire Farapuls ...
September 17, 2020 — Debates over whether hydroxychloroquine should be taken to help lessen the duration and impact of C ...
September 14, 2020 — Philips Healthcare released new imaging and workflow enhancements for its novel Kodex-EPD cardiac ...

 October 08, 2020
October 08, 2020