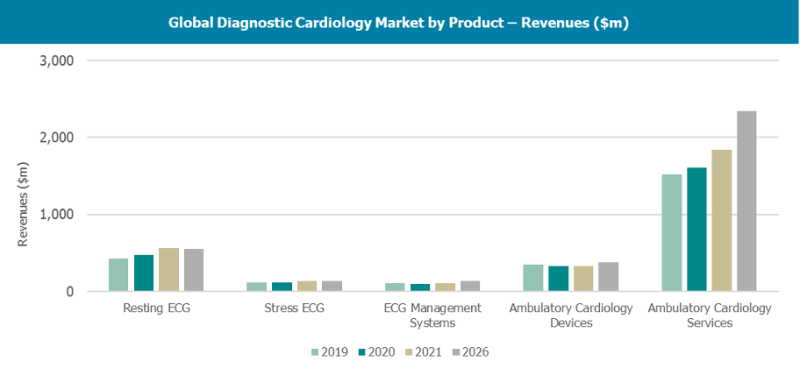
Like most healthcare markets, the diagnostic cardiology market has had a bumpy ride in recent years. The COVID-19 pandem ...
EP Lab
This channel includes news and new technology innovations for cardiac electrophysiology (EP) systems, techniques and devices using in EP labs. This includes implantable EP devices, pacemakers, implantable cardioverter defibrillators (ICD), cardiac resychronization therapy (CRT), ablation technologies, left atrial appendage (LAA) occlusion, atrial fibrilation (AF) and Holter monitors.
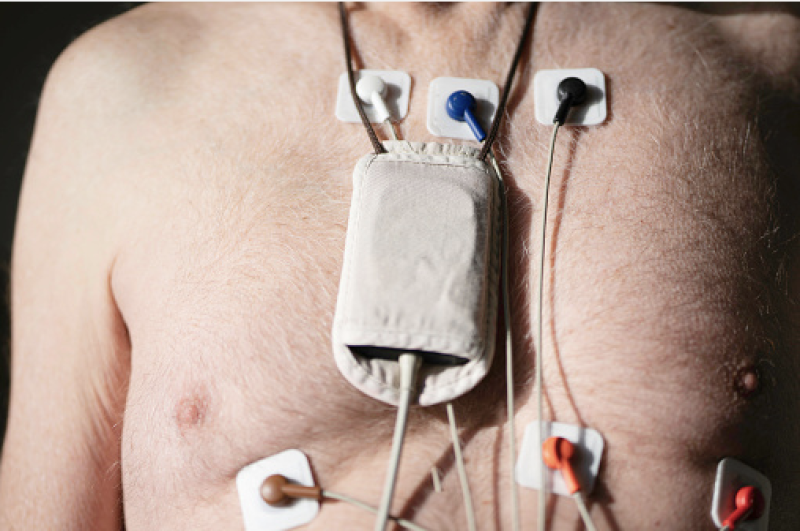
Holter monitors are firmly ensconced as the standard device for remote cardiac monitoring. But does that make them the ...
August 19, 2022 — According to a release just issued by the U.S. Food and Drug Administration (FDA), Medtronic is ...
When the patients of Michael Boler, M.D. need cardiac monitoring, the Holter monitor is no longer his first choice. “The ...
August 18, 2022 — A first-in-human multicenter trial involving Mayo Clinic used a new ablation technique for patients ...
August 17, 2022 — A new research paper was published in Aging (“Aging (Albany NY)” by Medline/PubMed, “Aging-US” by Web ...
August 5, 2022 — Black people and women with severe heart failure who might be good candidates for surgery to implant a ...
Apple created a stir when it announced in 2018 that its Apple Watch Series 4 was the first consumer health and fitness ...
August 4, 2022 — The Heart Rhythm Society (HRS) is a professional society representing a diverse population of ...
July 29, 2022 — A cohort study of persons with incident atrial fibrillation (AF) has found that AF after noncardiac ...
July 25, 2022 — iRhythm Technologies, Inc., a leading digital healthcare solutions company focused on the advancement ...
Washington Health System (WHS) provides healthcare services at more than 40 offsite locations across three counties in ...
July 22, 2022 — BioSig Technologies, Inc. a medical technology company advancing electrophysiology workflow by ...
July 21, 2022 — The global automated external defibrillators market size was valued at USD 717 million in 2021. It is ...
July 20, 2022 — CardioFocus, Inc., a medical device company dedicated to advancing ablation treatments for atrial ...
Apple created a stir when it announced in 2018 that its Apple Watch Series 4 was the first consumer health and fitness ...
July 13, 2022 — Adults with abnormal heart metabolism are up to three times more likely to experience life-threatening ...
July 11, 2022 — BioSig Technologies, Inc., a medical technology company advancing electrophysiology workflow by ...
July 6, 2022 — Atrial fibrillation (AF) is a prevalent cardiovascular condition with one of the highest rates of ...

 August 23, 2022
August 23, 2022