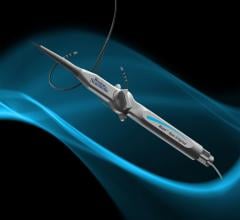
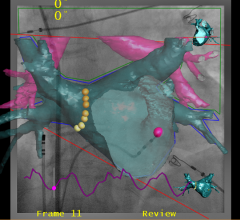
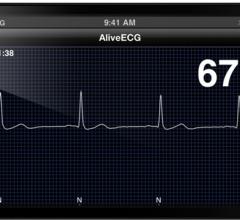
January 22, 2013 — Biotronik announced that the U.S. Food and Drug Administration (FDA) has approved, with conditions ...
EP Lab
This channel includes news and new technology innovations for cardiac electrophysiology (EP) systems, techniques and devices using in EP labs. This includes implantable EP devices, pacemakers, implantable cardioverter defibrillators (ICD), cardiac resychronization therapy (CRT), ablation technologies, left atrial appendage (LAA) occlusion, atrial fibrilation (AF) and Holter monitors.
January 21, 2013 — The first patient has been treated in the Boston Scientific Corporation ZERO AF clinical trial to ...
January 21, 2013 — At the Boston Atrial Fibrillation (AF) Symposium 2013, Philips Healthcare introduced its latest ...
When the patients of Michael Boler, M.D. need cardiac monitoring, the Holter monitor is no longer his first choice. “The ...
January 18, 2013 — nContact Inc. announced that the U.S. Food and Drug Administration (FDA) has cleared the Company’s ...
January 18, 2013 — CardioMEMS Inc. was honored as the 2012 recipient of the Intel Innovation Award. Founder and CEO Jay ...
January 16, 2013 — The U.S. Food and Drug Administration (FDA) has cleared St. Jude Medical’s new version of its Merlin ...
Apple created a stir when it announced in 2018 that its Apple Watch Series 4 was the first consumer health and fitness ...
January 16, 2013 — Medtronic’s CareLink Express service is a remote monitoring system that enables clinicians in ...
January 8, 2013 — The results of a recent study presented yesterday at the American Heart Association Scientific ...
January 4, 2013 — Biotronik announced that the European Heart Journal has published the clinical results of the ECOST tr ...
Washington Health System (WHS) provides healthcare services at more than 40 offsite locations across three counties in ...
January 3, 2013 — Tensys Medical Inc has received 510(k) clearance from the U.S. Food and Drug Administration (FDA) for ...
December 31, 2012 — St. Jude Medical Inc. (announced European CE mark approval of the Assura portfolio of implantable ...
December 28, 2012 — The first patient has been implanted with the Boston Scientific Corporation next generation Ingevity ...
Apple created a stir when it announced in 2018 that its Apple Watch Series 4 was the first consumer health and fitness ...
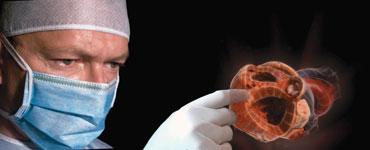
The annual Transcatheter Cardiovascular Therapeutics (TCT) meeting brings many cutting-edge technologies to the ...
December 21, 2012 — Nanostim Inc. announced the first successful implants of a leadless pacemaker in a series of 11 ...
December 19, 2012 — BioControl Medical has announced that the pilot clinical study of its CardioFit vagus nerve ...


 January 22, 2013
January 22, 2013