June 23, 2011 — A new study published in the June edition of the Journal of NeuroInterventional Surgery highlights that ...
Embolization devices
Emoblization devices allow the occlusion of selective blood vessels by introducing emboli.
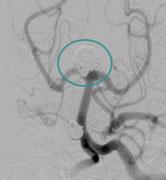
April 8, 2011 – The U.S. Food and Drug Administration (FDA) has approved an aneurysm treatment system as a ...
April 1, 2011 – A new minimally invasive embolization catheter was unveiled at the Society of Interventional ...
January 31, 2011 – The U.S. Food and Drug Administration (FDA) has approved a system for accessing and delivering ...
December 17, 2010 – The first truly detachable hydrogel polymer embolic device is now available nationwide. The ...
October 4, 2010 - Johnson and Johnson has completed the acquisition of Micrus Endovascular, a developer and ...
February 5, 2010 – Enrollment is complete for the trial of a polymer-based gel that rapidly transitions to a solid ...
July 17, 2009 – AGA Medical Corp. received European CE mark approval for its AMPLATZER vascular plug, AVP 4, which ...

Endovascular aneurysm repair (EVAR) using transcatheter-deployed stent grafts has grown significantly in the past 10 ...
Micrus Endovascular Corp. launched the Cerecyte and stretch-resistant versions of its DeltaPaq microcoil system ...
October 28, 2008 - The Los Angeles Brain and Spine Institute yesterday said George Rappard, M.D., a ...
September 23, 2008 - Micrus Endovascular Corp. today launched the Cerecyte and stretch-resistant versions of its ...
September 17, 2008 - Micrus Endovascular Corp. yesterday initiated its worldwide launch of Neuropath guide ...
July 11, 2008 - Micrus Endovascular Corp. said this week it received approval from the Ministry of Health, Labour ...
Terumo Interventional Systems offers the AZUR Peripheral HydroCoil Embolization System for the occlusion of blood ...


 June 23, 2011
June 23, 2011



