November 7, 2016 – A multicenter randomized trial evaluating the role of embolic protection using the Sentinel device ...
Embolic Protection Devices
This channel includes news and new technology innovations for embolic protection systems to prevent emboli from flowing down stream (distally) in arteries due to interventional or TAVR procedures.
September 29, 2016 — Keystone Heart Ltd. recently announced the safety and efficacy of the TriGuard Cerebral Embolic ...
September 20, 2016 – Claret Medical announced its filing of a marketing application with the U.S. Food and Drug ...

Atrial fibrillation (AF) affects nearly 6 million Americans and the condition puts them at significantly greater risk of ...

October 14, 2015 — Claret Medical, an innovator in cardiovascular cerebral protection, Data presented this week at ...
June 23, 2015 - InspireMD Inc. announced that its CGuard embolic prevention system reported positive results in the PARA ...
June 1, 2015 — Silk Road Medical Inc. announced the company received U.S. Food & Drug Administration (FDA) 510(k) ...
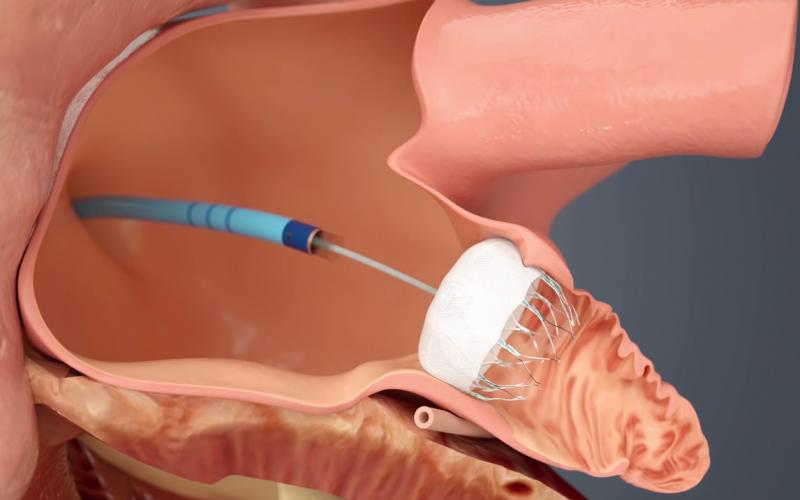
The long-awaited U.S. Food and Drug Administration (FDA) approval of the first transcatheter left atrial appendage (LAA) ...
April 20, 2015 — Contego Medical announced the completion of a $5.6 million Series B financing round for the development ...

March 19, 2015 — An investigational device that deflects debris away from the brain during transcatheter aortic valve ...
January 21, 2015 — When retrievable inferior vena cava (IVC) filters were approved for use in the United States in 2003 ...

January 5, 2015 — The first large-scale, multispecialty prospective clinical research trial to evaluate the use of infer ...
December 31, 2014 — Innovative Cardiovascular Solutions LLC has completed a Class A Unit financing totaling $5 million ...
By Dave Fornell, editor of DAIC Magazine
The key take away messages from the 26th annual Transcatheter Cardiovascular ...


 November 07, 2016
November 07, 2016



