Anthony Chang, M.D., chief intelligence and innovation officer, Children's Hospital of Orange County (CHOC), and medical ...
Diagnostic and Interventional Cardiology DAIC
Diagnostic and Interventional Cardiology (DAIC) is a magazine that reaches more than 25,000 healthcare professionals in cardiology, interventional cardiology and cath labs across the United States. These influential buying team members rely on DAIC's award-winning editorial content and comparison charts as a unique research tool for specifying, recommending and approving technology/device purchases.
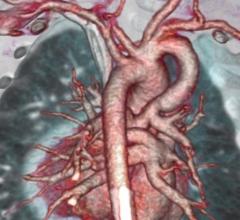
March 4, 2019 — Artificial intelligence (AI) radiology solution provider Aidoc announced the commercial release of its ...

March 1, 2019 — When it comes to treatment options for a damaged heart valve, nothing is getting more attention than tra ...
Robert Klempfner, M.D., director of the Cardiovascular Prevention Institute, Sheba Medical Center, Israel, discusses ...
February 28, 2019 — Obesity and severe obesity in childhood and adolescence have been added to the list of conditions ...
How wearable devices will play a role in healthcare was a big topic at the Healthcare Information Management and Systems ...
February 25, 2019 — Diagnostic and Interventional Cardiology has been recognized as a finalist in the Jesse H. Neal ...
February 25, 2019 — GE Healthcare announced Feb. 25 the sale of its biopharmaceutical business to Danaher, and GE ...
Here is what I thought were the top five most important take-away presentations from the 2019 Society of Thoracic ...
February 25, 2019 — Philips will unveil a new mixed reality concept developed together with Microsoft that the company ...
February 21, 2019 — At the recent Healthcare Information and Management Systems Society (HIMSS) annual conference, AT&T ...
Clinical study data makes the world go around in cardiology and is the basis of setting guidelines in evidence-based ...
February 20, 2019 — California-based Vascular Dynamics Inc. (VDI) is sponsoring a new clinical trial, called CALM-2 ...
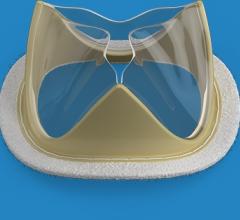
February 18, 2019 — Foldax Inc. announced the U.S. Food and Drug Administration (FDA) has granted investigative device ...

 March 04, 2019
March 04, 2019