October 5, 2016 — Vascular Solutions Inc. initiated a nationwide recall of its Twin-Pass Dual Access catheters used in ...
Catheters
August 10, 2016 — Mercator MedSystems Inc. announced the enrollment of the first critical limb ischemia (CLI) patients ...
August 10, 2016 — Access Scientific introduced the PowerWand XL, a companion to the PowerWand All-in-One, in July. The ...
July 13, 2016 — VentureMed Group Ltd., specializing in devices for the endovascular treatment of peripheral arterial ...
January 7, 2016 — Roxwood Medical Inc. reported that more than 500 patients have been successfully treated as part of ...
December 28, 2015 — The U.S. Food and Drug Administration (FDA) said Boston Scientific has started a Class I recall of ...
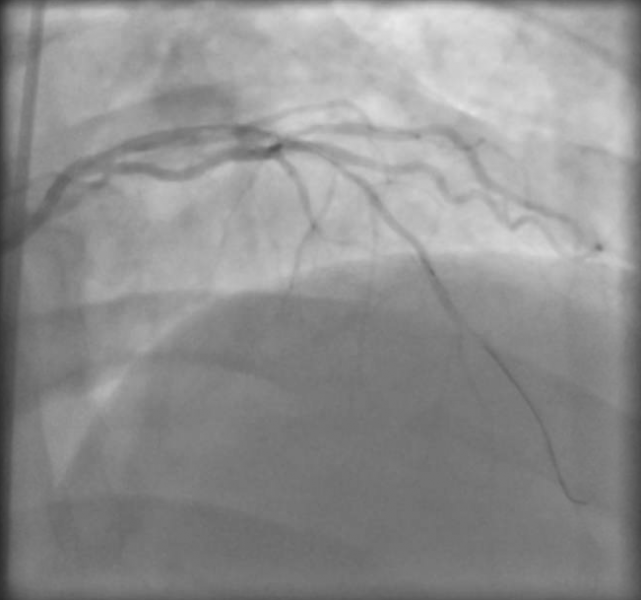
November 24, 2015 — The U.S. Food and Drug Administration (FDA) issued a warning this week that hydrophilic and/or ...
Todd Brinton, M.D., clinical associate professor and consulting associate professor of bioengineering at Stanford ...
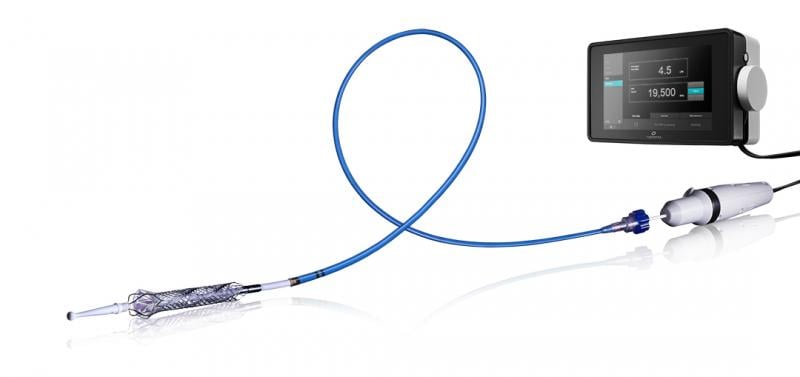
July 15, 2015 - Thoratec announced that the HeartMate PHP (Percutaneous Heart Pump) received CE Mark approval ...
March 3, 2015 — A study being presented at the Society of Interventional Radiology’s annual scientific meeting says 3-D ...
December 17, 2014 — ReFlow Medical Inc. announced U.S. Food and Drug Administration (FDA) clearance for ...
October 2, 2014 — ReFlow Medical Inc. announced the initial clinical use of its Wingman35 Crossing Catheter and speX ...
July 30, 2014 — Covidien announced the commercial launch of its next-generation Trellis peripheral infusion system. The ...


 October 05, 2016
October 05, 2016