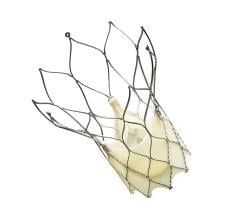
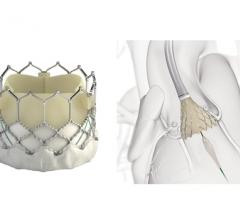
October 1, 2018 — Boston Scientific announced that the U.S. Food and Drug Administration (FDA) has approved its ...
Cath Lab
The catheterization lab channel includes content related to interventional technologies for coronary and peripheral artery disease (PAD). Other cath lab transcatheter device technologies covered on this page included percutaneous treatment for stroke, venous interventions, heart valves, hypertension, heart failure and percutenous coronary interventions (PCI).
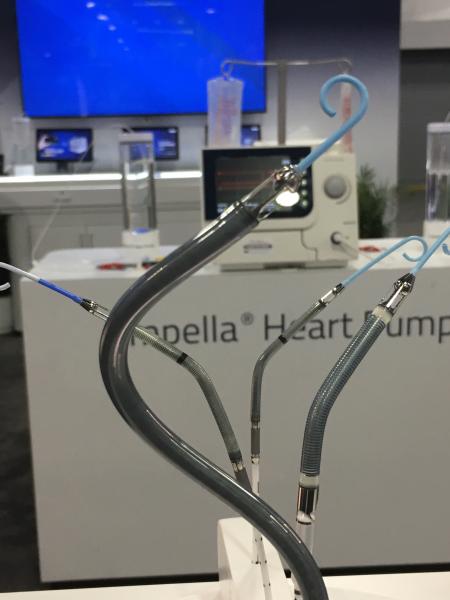
October 1, 2018 — Here is the list of the most popular content on the Diagnostic and Interventional Cardiology (DAIC) ...
October 1, 2018 — New results from the RENASCENT studies presented at the Transcatheter Cardiovascular Therapeutics (TCT ...
Adjustable parameters on the ACIST® CVI® Contrast Delivery System include, flow rate, volume, pressure limit, rise time ...
Nevin Kapur, M.D., FAHA, FACC, FSCAI, executive director, Cardiovascular Center for Research and Innovation, Tufts ...
September 28, 2018 — Heart failure patients who received the Carillon Mitral Contour System in the REDUCE FMR clinical ...
September 28, 2018 — At one year following transcatheter aortic valve replacement (TAVR), implantation with the Portico ...
As medical advancements continue to push the boundaries of what is possible in the field of structural heart ...
September 28, 2018 — The first randomized study to compare general versus local anesthesia during transcatheter aortic ...
Philippe Genereux, M.D., co-director of the structural heart program at the Gagnon Cardiovascular Institute at ...
September 27, 2018 — Biotronik recently announced U.S. Food and Drug Administration (FDA) approval of the PK Papyrus ...
While the current positive revolution in percutaneous coronary intervention (PCI) practice has been made possible by the ...
Akshay Khandelwal, M.D., director of medical operations at the Henry Ford Heart and Vascular Institute, Detroit, and ...
William Abraham, M.D., FACC, professor of medicine and director of the division of cardiovascular medicine, The Ohio ...
Ron Waksman, M.D., associate director of the division of cardiology and director of cardiovascular research and advanced ...
During cardiology fellowship, my institution had a hand manifold system. I found it difficult to use at times, and it ...
September 26, 2018 — Patients with heart failure and secondary mitral regurgitation (MR) who remained symptomatic ...
September 26, 2018 — Positive 12-month data from the late-breaking IMPERIAL trial was presented at the 2018 ...
September 25, 2018 – New data demonstrate the correlation between the presence of non-flow-limiting, non-intervened-upon ...


 October 01, 2018
October 01, 2018