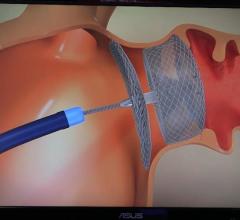
October 4, 2018 — The Ohio State University Wexner Medical Center is first in the country to test a new medical device ...
Cath Lab
The catheterization lab channel includes content related to interventional technologies for coronary and peripheral artery disease (PAD). Other cath lab transcatheter device technologies covered on this page included percutaneous treatment for stroke, venous interventions, heart valves, hypertension, heart failure and percutenous coronary interventions (PCI).
October 4, 2018 — The late-breaking FAST-FFR Trial demonstrated that the sensitivity and specificity of the CathWorks ...
October 4, 2018 — Pulmonary artery denervation (PADN) is associated with significant improvements in hemodynamic and ...
Adjustable parameters on the ACIST® CVI® Contrast Delivery System include, flow rate, volume, pressure limit, rise time ...
October 4, 2018 — Three-dimensional printing company Biomodex, which develops organ twins for advanced physician trainin ...
October 4, 2018 – Investigators unveiled clinical data from the independent BIONYX and SORT OUT IX all-comers trials ...
DAIC Editor Dave Fornell takes a tour of some of the most innovative new cardiovascular technology he found on the expo ...
As medical advancements continue to push the boundaries of what is possible in the field of structural heart ...
October 3, 2018 — Image Diagnostics has entered into a product development agreement with ECLS Inc. to design a new radi ...

One of the key, early pioneers who helped bring coronary angioplasty to the United States in the late 1970s was Simon ...
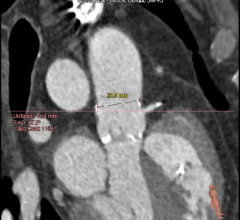
October 3, 2018 — A high number of patients in a study who underwent transcatheter aortic valve replacement (TAVR) exper ...
While the current positive revolution in percutaneous coronary intervention (PCI) practice has been made possible by the ...
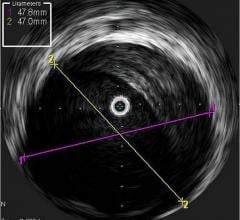
October 2, 2018 — Intravascular ultrasound (IVUS) guidance improved clinical outcomes over angiography guidance during d ...
Cindy Grines, M.D., chair and professor, department of cardiology, Zucker School of Medicine, Hostra/Northwell, spoke on ...
Ashish Pershad, M.D., medical director, structural heart program, Banner University Medical Heart Institute, Phoenix ...
During cardiology fellowship, my institution had a hand manifold system. I found it difficult to use at times, and it ...
October 1, 2018 — Recent results from the BIONYX randomized clinical study showed the novel, thin-strutted, polymer ...
October 1, 2018 — Boston Scientific announced that the U.S. Food and Drug Administration (FDA) has approved its ...
October 1, 2018 — Here is the list of the most popular content on the Diagnostic and Interventional Cardiology (DAIC) ...


 October 04, 2018
October 04, 2018