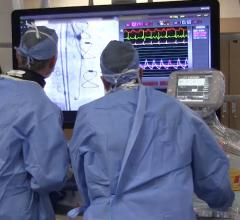
John Carroll, M.D., FACC, FSCAI, director of interventional cardiology, and Robert Quaife, M.D., director of advanced ...
Cath Lab
The catheterization lab channel includes content related to interventional technologies for coronary and peripheral artery disease (PAD). Other cath lab transcatheter device technologies covered on this page included percutaneous treatment for stroke, venous interventions, heart valves, hypertension, heart failure and percutenous coronary interventions (PCI).

January 4, 2018 — Here is the top cardiology content on the Diagnostic and Interventional Cardiology (DAIC) website from ...
Interview with John Messenger, M.D., FACC, FSCAI, director of the cardiac cath labs and director of the cardiovascular ...
Adjustable parameters on the ACIST® CVI® Contrast Delivery System include, flow rate, volume, pressure limit, rise time ...
January 3, 2019 — Ohio University and the National Academy of Engineering announced the 2019 Fritz J. and Dolores H ...
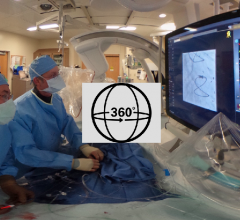
This is a 360 degree photo during a chronic total occlusion (CTO) procedure at the University of Colorado Hospital with ...
This is a walk through inside one of the cardiac hybrid cath labs at the University of Colorado Hospital in Aurora ...
As medical advancements continue to push the boundaries of what is possible in the field of structural heart ...
This is a quick video tour of one of the dedicated electrophysiology (EP) labs at the University of Colorado Hospital ...
This is the newest cardiac cath lab at the University of Colorado Hospital in Aurora, Colorado. Construction was ...
January 2, 2019 — Boston Scientific Corp. exercised its option to acquire the remaining shares of Millipede Inc, a ...
While the current positive revolution in percutaneous coronary intervention (PCI) practice has been made possible by the ...
December 27, 2018 — Patients who arrive at the hospital with heart-attack-like symptoms have had little recourse for ...
December 21, 2018 — CathWorks announced that its FFRangio System received U.S. Food and Drug Administration (FDA) ...
John Carroll, M.D., FACC, FSCAI, director of interventional cardiology at the Cardiac and Vascular Center at the Univers ...
During cardiology fellowship, my institution had a hand manifold system. I found it difficult to use at times, and it ...
Andreas Brieke, M.D., director of mechanical circulatory support, heart failure physician and site principal ...
December 20, 2018 — Cardiva Medical Inc. announced the company has received premarket approval (PMA) from the U.S. Food ...
December 19, 2018 — A new set of appropriate use criteria (AUC) released Dec. 17 by a group of cardiovascular ...

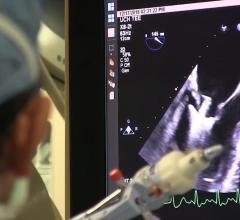
 January 07, 2019
January 07, 2019