January 4, 2018 — Here is the top cardiology content on the Diagnostic and Interventional Cardiology (DAIC) website from ...
Cath Lab
The catheterization lab channel includes content related to interventional technologies for coronary and peripheral artery disease (PAD). Other cath lab transcatheter device technologies covered on this page included percutaneous treatment for stroke, venous interventions, heart valves, hypertension, heart failure and percutenous coronary interventions (PCI).
Interview with John Messenger, M.D., FACC, FSCAI, director of the cardiac cath labs and director of the cardiovascular ...
January 3, 2019 — Ohio University and the National Academy of Engineering announced the 2019 Fritz J. and Dolores H ...
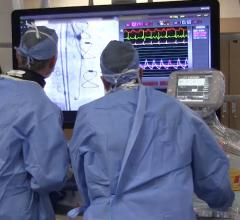
Adjustable parameters on the ACIST® CVI® Contrast Delivery System include, flow rate, volume, pressure limit, rise time ...
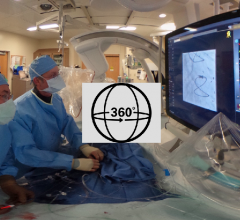
This is a 360 degree photo during a chronic total occlusion (CTO) procedure at the University of Colorado Hospital with ...
This is a walk through inside one of the cardiac hybrid cath labs at the University of Colorado Hospital in Aurora ...
This is a quick video tour of one of the dedicated electrophysiology (EP) labs at the University of Colorado Hospital ...
As medical advancements continue to push the boundaries of what is possible in the field of structural heart ...
This is the newest cardiac cath lab at the University of Colorado Hospital in Aurora, Colorado. Construction was ...
January 2, 2019 — Boston Scientific Corp. exercised its option to acquire the remaining shares of Millipede Inc, a ...
December 27, 2018 — Patients who arrive at the hospital with heart-attack-like symptoms have had little recourse for ...
While the current positive revolution in percutaneous coronary intervention (PCI) practice has been made possible by the ...
December 21, 2018 — CathWorks announced that its FFRangio System received U.S. Food and Drug Administration (FDA) ...
John Carroll, M.D., FACC, FSCAI, director of interventional cardiology at the Cardiac and Vascular Center at the Univers ...
Andreas Brieke, M.D., director of mechanical circulatory support, heart failure physician and site principal ...
During cardiology fellowship, my institution had a hand manifold system. I found it difficult to use at times, and it ...
December 20, 2018 — Cardiva Medical Inc. announced the company has received premarket approval (PMA) from the U.S. Food ...
December 19, 2018 — A new set of appropriate use criteria (AUC) released Dec. 17 by a group of cardiovascular ...
December 18, 2018 — Merit Medical Systems Inc. announced that it has acquired substantially all of the assets of ...


 January 04, 2019
January 04, 2019