February 1, 2019 — Merit Medical Systems Inc. announced that the PreludeSync Distal Compression Device is now available ...
Cath Lab
The catheterization lab channel includes content related to interventional technologies for coronary and peripheral artery disease (PAD). Other cath lab transcatheter device technologies covered on this page included percutaneous treatment for stroke, venous interventions, heart valves, hypertension, heart failure and percutenous coronary interventions (PCI).
January 31, 2019 — Cardiovascular Systems Inc. announced that the first patients in the United States were treated using ...

The higher expense and lower frame rates of 3-D cardiac ultrasound systems have limited their adoption over the past ...
Adjustable parameters on the ACIST® CVI® Contrast Delivery System include, flow rate, volume, pressure limit, rise time ...
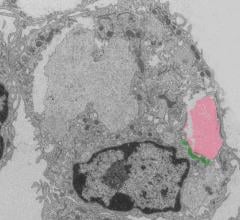
January 30, 2019 — Northwestern Medicine scientists have discovered a novel signaling pathway that promotes healing ...

In recent years, there has been a lot of focus by vendors on developing better stenting technologies to treat peripheral ...
January 30, 2019 — XableCath Inc., announced that its XableCath blunt and abrasion tip catheters were cleared by the U.S ...
As medical advancements continue to push the boundaries of what is possible in the field of structural heart ...
January 29, 2019 — Thubrikar Aortic Valve Inc. announced the first human implant of the Optimum TAV using their transcat ...

There was a lot of hype and high hopes pinned on bioresorbable stent technologies as the way of the future two years ago ...

There are a few recent trends in X-ray angiography imaging systems that hospitals should be aware of if they are looking ...
While the current positive revolution in percutaneous coronary intervention (PCI) practice has been made possible by the ...
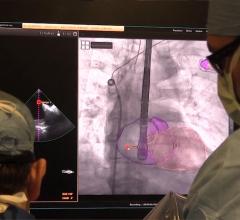
January 28, 2019 — Lenox Hill Hospital is the first hospital in New York City to successfully implant Abbott’s Tendyne t ...
January 28, 2019 — Philips announced the latest pooled analysis of patient-level data of over 2,300 patients treated ...
DAIC Editor Dave Fornell takes a tour of some of the most interesting new medical imaging technologies displayed on the ...
During cardiology fellowship, my institution had a hand manifold system. I found it difficult to use at times, and it ...

The anti-proliferative drug paclitaxel has been used as a coating on coronary stents to prevent restenosis since 2003 ...
Joe Cleveland, M.D., professor of cardiothoracic surgery, at the University of Colorado Hospital, offers a cardiac ...

Catheter-based blood clot removal a decade ago was a standard of care for acute coronary revascularization, but declined ...


 February 01, 2019
February 01, 2019