April 3, 2019 — The U.S. Food and Drug Administration (FDA) recently cleared Bard Peripheral Vascular's Venovo Venous ...
Cath Lab
The catheterization lab channel includes content related to interventional technologies for coronary and peripheral artery disease (PAD). Other cath lab transcatheter device technologies covered on this page included percutaneous treatment for stroke, venous interventions, heart valves, hypertension, heart failure and percutenous coronary interventions (PCI).
April 3, 2019 — Essential Medical Inc. received U.S. Food and Drug Administration (FDA) clearance for its large bore ...
April 3, 2019 — Medtronic's Resolute Integrity Zotarolimus-eluting Coronary Stent System received an additional U.S ...
Adjustable parameters on the ACIST® CVI® Contrast Delivery System include, flow rate, volume, pressure limit, rise time ...

Interview with Frederick Masoudi, M.D., FACC, FAHA, professor of cardiology at the University of Colorado Hospital, and ...
As medical advancements continue to push the boundaries of what is possible in the field of structural heart ...
March 27, 2019 — Training interventional radiologists to perform endovascular thrombectomies results in positive ...
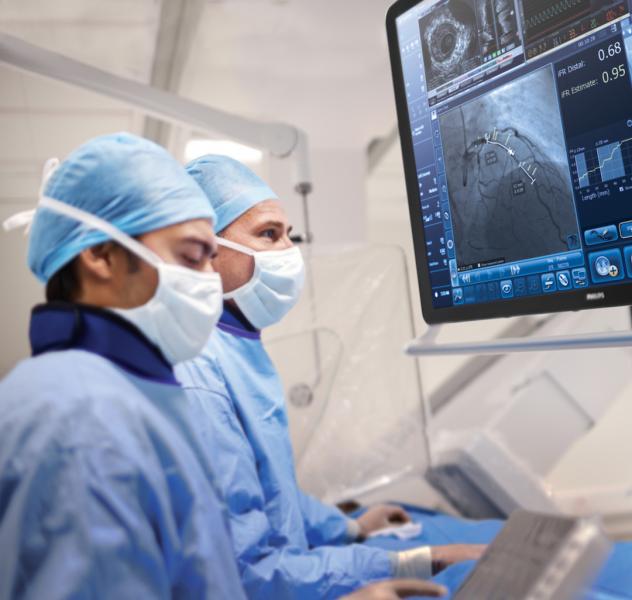
March 27. 2019 — A newly released expert consensus statement provides recommendations for the safe and effective ...
While the current positive revolution in percutaneous coronary intervention (PCI) practice has been made possible by the ...
March 27, 2019 — The Centers for Medicare and Medicaid Services (CMS) proposed to update its national coverage policy ...

Right heart failure (RHF) is a syndrome characterized by the inability of the right ventricle (RV) to support optimal ...
March 26, 2019 — Level Ex, creators of medical video games for physicians, expanded into cardiology with the launch of ...
During cardiology fellowship, my institution had a hand manifold system. I found it difficult to use at times, and it ...
March 25, 2019 — Patients who stopped taking aspirin one month after receiving a stent in the heart’s arteries but ...
March 25, 2019 — NZ Technologies Inc. announced the first published clinical review on its TIPSO technology’s ability to ...
March 22, 2019 — Among patients with severe symptomatic aortic stenosis at low surgical risk, transcatheter aortic valve ...


 April 03, 2019
April 03, 2019