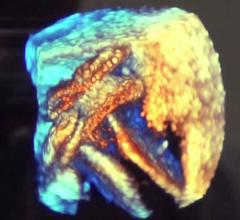
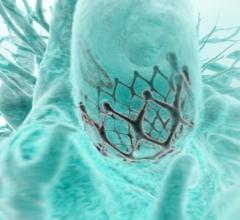
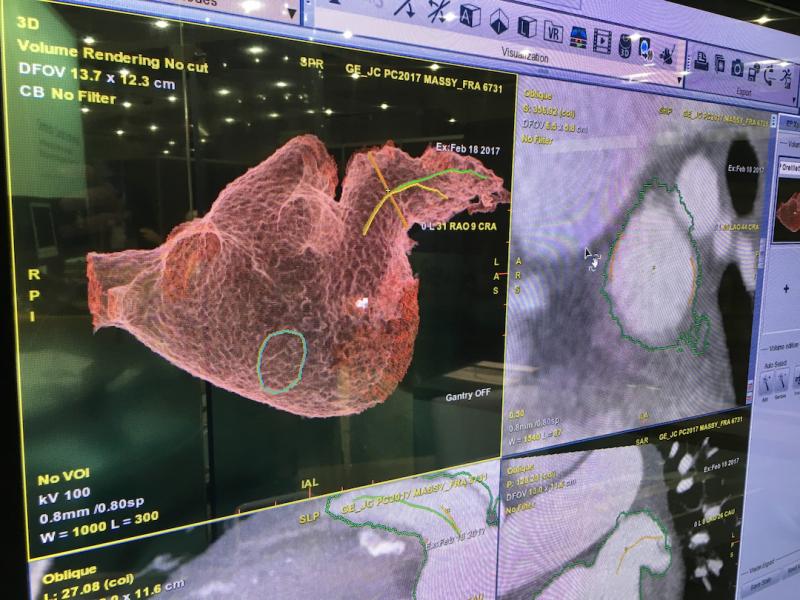
April 3, 2019 – The ACC.19 late-breaking landmark Evolut Low Risk Trial compared the minimally invasive Evolut ...
Cath Lab
The catheterization lab channel includes content related to interventional technologies for coronary and peripheral artery disease (PAD). Other cath lab transcatheter device technologies covered on this page included percutaneous treatment for stroke, venous interventions, heart valves, hypertension, heart failure and percutenous coronary interventions (PCI).
April 3, 2019 — The U.S. Food and Drug Administration (FDA) recently granted an additional indication to Bard Peripheral ...
April 3, 2019 — The U.S. Food and Drug Administration (FDA) recently cleared Bard Peripheral Vascular's Venovo Venous ...
Adjustable parameters on the ACIST® CVI® Contrast Delivery System include, flow rate, volume, pressure limit, rise time ...
April 3, 2019 — Essential Medical Inc. received U.S. Food and Drug Administration (FDA) clearance for its large bore ...
April 3, 2019 — Medtronic's Resolute Integrity Zotarolimus-eluting Coronary Stent System received an additional U.S ...

As medical advancements continue to push the boundaries of what is possible in the field of structural heart ...
Interview with Frederick Masoudi, M.D., FACC, FAHA, professor of cardiology at the University of Colorado Hospital, and ...
March 27, 2019 — Training interventional radiologists to perform endovascular thrombectomies results in positive ...
While the current positive revolution in percutaneous coronary intervention (PCI) practice has been made possible by the ...
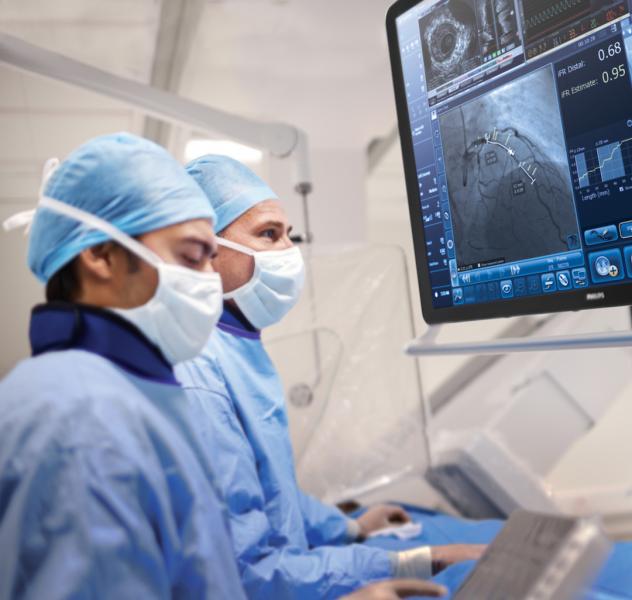
March 27. 2019 — A newly released expert consensus statement provides recommendations for the safe and effective ...
March 27, 2019 — The Centers for Medicare and Medicaid Services (CMS) proposed to update its national coverage policy ...
During cardiology fellowship, my institution had a hand manifold system. I found it difficult to use at times, and it ...

Right heart failure (RHF) is a syndrome characterized by the inability of the right ventricle (RV) to support optimal ...
March 26, 2019 — Level Ex, creators of medical video games for physicians, expanded into cardiology with the launch of ...
March 25, 2019 — Patients who stopped taking aspirin one month after receiving a stent in the heart’s arteries but ...

 April 03, 2019
April 03, 2019