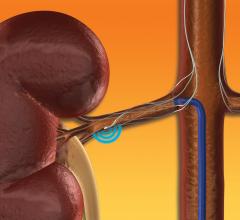
June 10, 2019 – W. L. Gore & Associates (Gore) announced the U.S. Food and Drug Administration’s (FDA’s) premarket ...
Cath Lab
The catheterization lab channel includes content related to interventional technologies for coronary and peripheral artery disease (PAD). Other cath lab transcatheter device technologies covered on this page included percutaneous treatment for stroke, venous interventions, heart valves, hypertension, heart failure and percutenous coronary interventions (PCI).
A discussion with Nicholas Bevins, Ph.D., vice chair, physics and research, and Jessica Harrington, RCIS. They explain ...
June 4, 2019 — Royal DSM recently announced a collaboration with Strait Access Technologies (SAT), to develop the world ...
Adjustable parameters on the ACIST® CVI® Contrast Delivery System include, flow rate, volume, pressure limit, rise time ...
June 4, 2019 — Medis Medical Imaging Systems B.V. has received clearance from the U.S. Food and Drug Administration for ...
May 31, 2019 — Terumo Medical Corp. is recalling the SoloPath Balloon Expandable TransFemoral System and Re-Collapsible ...
May 29, 2019 — Philips announced the three-year results from the ILLUMENATE Pivotal trial and the ILLUMENATE European ...
As medical advancements continue to push the boundaries of what is possible in the field of structural heart ...
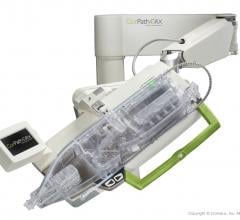
May 28, 2019 — Corindus Vascular Robotics Inc. announced its CorPath GRX System was successfully used to perform a live ...
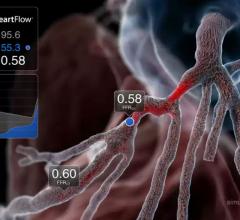
May 28, 2019 — New data demonstrated that use of the investigational HeartFlow Planner, a real-time, non-invasive ...
Physicians use many strategies to better interface with patients and their families to try and explain in non-physician ...
While the current positive revolution in percutaneous coronary intervention (PCI) practice has been made possible by the ...
May 23, 2019 — People who skip breakfast and eat dinner near bedtime have worse outcomes after a heart attack, according ...
May 23, 2019 – Investigators unveiled late-breaking clinical data from a first-of-its-kind physician sponsored clinical ...
May 23, 2019 — ControlRad Inc. announced that the U.S. Food and Drug Administration (FDA) granted 510(k) clearance for ...
During cardiology fellowship, my institution had a hand manifold system. I found it difficult to use at times, and it ...
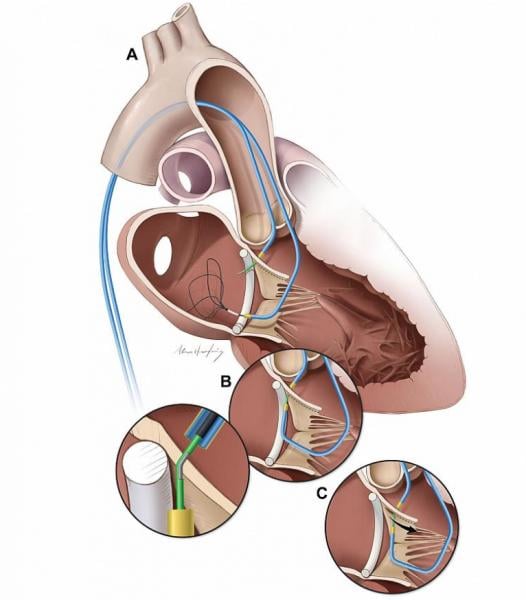
Researchers at the National, Heart, Lung, and Blood Institute (NHLBI), part of the National Institutes of Health (NIH) ...
May 22, 2019 — Abiomed announced that the Impella CP with SmartAssist will be commercially available beginning at the 20 ...
May 22, 2019 — Edwards Lifesciences Corp. announced strategic clinical and regulatory milestones for its Edwards Pascal ...


 June 10, 2019
June 10, 2019