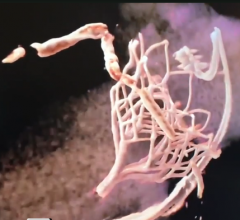
July 25, 2019 — Penumbra announced U.S. commercial availability of the Penumbra System’s most advanced technology, the ...
Cath Lab
The catheterization lab channel includes content related to interventional technologies for coronary and peripheral artery disease (PAD). Other cath lab transcatheter device technologies covered on this page included percutaneous treatment for stroke, venous interventions, heart valves, hypertension, heart failure and percutenous coronary interventions (PCI).

One of the big trends in cardiac computed tomography (CT) imaging has been the introduction of noninvasive fractional ...
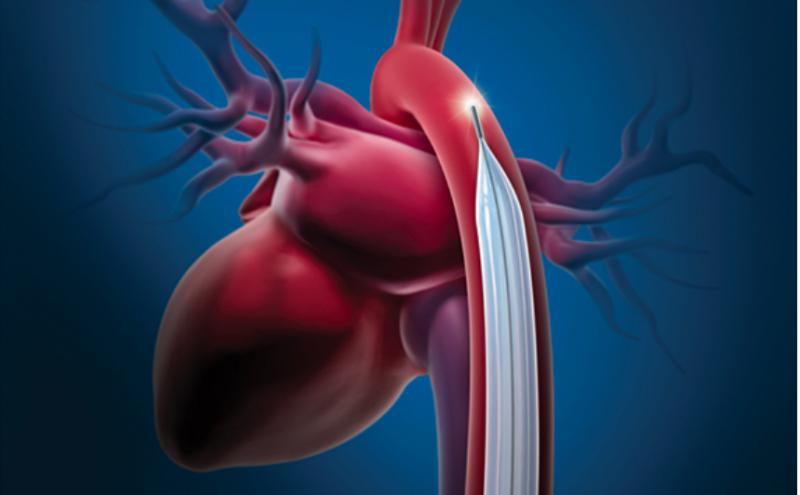
July 24, 2019 — The U.S. Food and Drug Administration announced Maquet/Datascope is recalling all intra-aortic balloon ...
Adjustable parameters on the ACIST® CVI® Contrast Delivery System include, flow rate, volume, pressure limit, rise time ...
July 24, 2019 — The West Virginia University (WVU) Heart and Vascular Institute is the first hospital in the country to ...
July 19, 2019 — Medical device startup company Filterlex Medical recently completed a series A financing round, raising ...
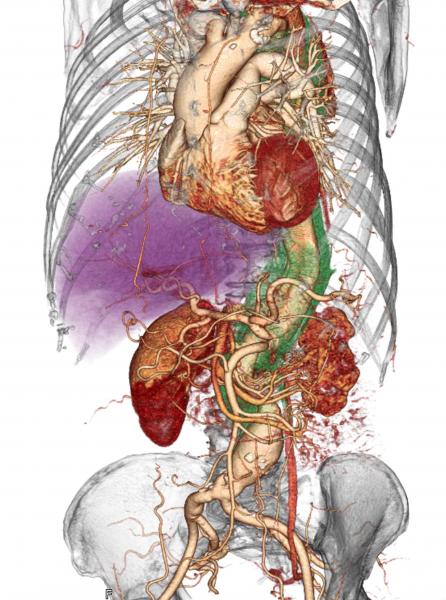
July 18, 2019 — When a patient comes to the hospital with acute type A aortic dissection (ATAAD), a tearing of the inner ...
As medical advancements continue to push the boundaries of what is possible in the field of structural heart ...
July 17, 2019 — Abbott has received U.S. Food and Drug Administration (FDA) approval for the most advanced MitraClip hea ...
July 15, 2019 — Edwards LifeSciences is recalling the IntraClude Intra-Aortic Occlusion Device due to a risk of balloon ...
July 11, 2019 — Mednax Inc. and Mednax Radiology Solutions announced that Chief Medical Officer Ricardo C. Cury, M.D ...
While the current positive revolution in percutaneous coronary intervention (PCI) practice has been made possible by the ...
July 10, 2019 — W. L. Gore & Associates Inc. (Gore) announced the first U.S. implant of the Gore Tag Conformable ...
July 9, 2019 — Neovasc Inc. announced that its Tiara transcatheter mitral valve replacement (TMVR) device and its ...
July 5, 2019 — FEops announced that FEops HEARTguide was the winning entry at CSI Frankfurt 2019 voted on by physician ...
During cardiology fellowship, my institution had a hand manifold system. I found it difficult to use at times, and it ...
July 3, 2019 — A study of a procedure developed at Henry Ford Health System shows inducing a heart attack in patients ...
Federico Asch, M.D., FASE, director of cardiac imaging research and director of the cardiovascular imaging lab, MedStar ...
Thomas Porter, M.D., FASE, the Theordore F. Hubbard Distinguished Chair of Cardiology and a professor of medicine at the ...

 July 25, 2019
July 25, 2019