September 5, 2019 — Abbott announced the launch of the company's TRILUMINATE Pivotal trial evaluating the safety and ...
Cath Lab
The catheterization lab channel includes content related to interventional technologies for coronary and peripheral artery disease (PAD). Other cath lab transcatheter device technologies covered on this page included percutaneous treatment for stroke, venous interventions, heart valves, hypertension, heart failure and percutenous coronary interventions (PCI).
September 3, 2019 — Shockwave Medical Inc. has received Breakthrough Device Designation from the U.S. Food and Drug ...
September 3, 2019 — Corindus Vascular Robotics Inc. announced that EClinicalMedicine, a clinical journal published by Th ...
Adjustable parameters on the ACIST® CVI® Contrast Delivery System include, flow rate, volume, pressure limit, rise time ...
September 3, 2019 — New Zealand-based Adept Medical announced the launch of the Antegrade IR Platform. Clinically driven ...

September 3, 2019 — Here is the list of the most popular content on the Diagnostic and Interventional Cardiology (DAIC) ...
August 29, 2019 – Merit Medical Systems Inc. announced the U.S. commercial launch of the PreludeSync Evo radial ...
As medical advancements continue to push the boundaries of what is possible in the field of structural heart ...

August 28, 2019 — The U.S. Food and Drug Administration (FDA) released an updated MedWatch Alert this month on the ...
August 28, 2019 — AtriCure Inc. announced it has received U.S. Food and Drug Administration (FDA) 510(k) clearance of ...
August 27, 2019 — Imagine there were a drug that you could take soon after a heart attack that could reduce damage by ...
While the current positive revolution in percutaneous coronary intervention (PCI) practice has been made possible by the ...
August 27, 2019 — Concept Medical was recently granted Breakthrough Therapy designation by the U.S. Food and Drug ...
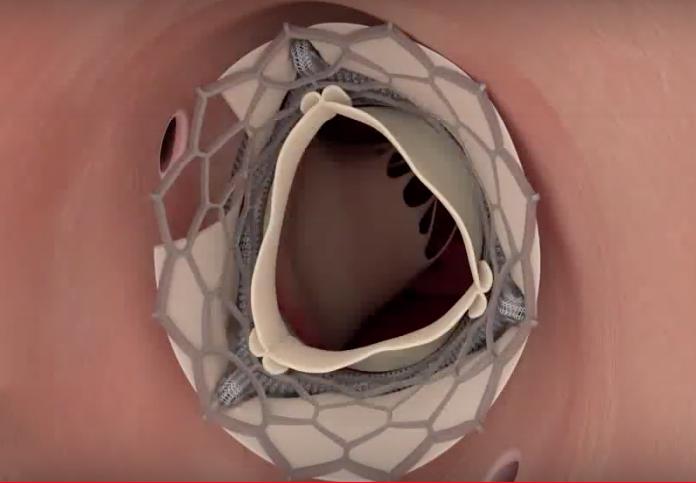
A novel technique has proven successful in preventing coronary artery obstruction during transcatheter aortic valve ...
August 26, 2019 — Areas with a higher number of fast food restaurants have more heart attacks, according to research ...
During cardiology fellowship, my institution had a hand manifold system. I found it difficult to use at times, and it ...
August 23, 2019 — Cook Medical recently released the second generation of the 2.6 Fr CXI Support Catheter with platinum ...
John Carroll, M.D., FACC, FSCAI, director of interventional cardiology at the Cardiac and Vascular Center at the ...
Interview with Kevin Rogers, M.D., director of vascular medicine at the University of Colorado Hospital. He explains ...

 September 05, 2019
September 05, 2019