September 20, 2019 – Abbott announced approvals in Europe for two of its pediatric devices — the Masters HP 15mm ...
Cath Lab
The catheterization lab channel includes content related to interventional technologies for coronary and peripheral artery disease (PAD). Other cath lab transcatheter device technologies covered on this page included percutaneous treatment for stroke, venous interventions, heart valves, hypertension, heart failure and percutenous coronary interventions (PCI).
September 20, 2019 — BioTrace Medical Inc. announced the company’s key activities at the 31st annual Transcatheter ...
September 20, 2019 — Orchestra BioMed Inc., in partnership with Terumo Corp. announced the company has secured ...
Adjustable parameters on the ACIST® CVI® Contrast Delivery System include, flow rate, volume, pressure limit, rise time ...
September 18, 2019 — Early use of an implantable cardioverter-defibrillator (ICD) after primary coronary intervention ...
September 17, 2019 — The European Society of Cardiology (ESC) published new guidelines on the diagnosis and management ...
September 17, 2019 — The U.S. Food and Drug Administration (FDA) has cleared the Artis icono, a high-precision family of ...
As medical advancements continue to push the boundaries of what is possible in the field of structural heart ...
A discussion with Khaldoon Alaswad, M.D., director, cardiac catheterization lab, Henry Ford Hospital, on treating ...
September 13, 2019 — Rex Medical L.P. has received 510(k) clearance from the U.S. Food and Drug Administration (FDA) for ...
September 12, 2019 — Corvia Medical has sponsored and is actively enrolling patients in a heart failure (HF) device ...
While the current positive revolution in percutaneous coronary intervention (PCI) practice has been made possible by the ...
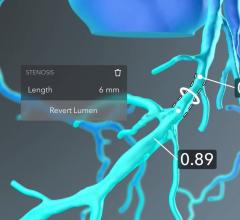
September 12, 2019 — HeartFlow Inc. has obtained clearance from the U.S. Food and Drug Administration (FDA) for the ...
A discussion with William O'Neill, M.D., director of the structural heart program, Henry Ford Hospital, and Michele ...
September 11, 2019 — Percutaneous reduction of secondary mitral regurgitation in patients with heart failure does not ...
During cardiology fellowship, my institution had a hand manifold system. I found it difficult to use at times, and it ...
September 10, 2019 — An international randomized trial has shown that complete revascularization reduces major ...

Primary percutaneous coronary intervention (PCI) is the preferred treatment for acute ST-segment elevation myocardial ...
September 5, 2019 — Biotronik's ultrathin Orsiro stent demonstrated superiority over Xience with respect to target ...


 September 20, 2019
September 20, 2019