Chandan Devireddy, M.D., offers insights about what he saw as the top take aways from the 2019 Transcatheter ...
Cath Lab
The catheterization lab channel includes content related to interventional technologies for coronary and peripheral artery disease (PAD). Other cath lab transcatheter device technologies covered on this page included percutaneous treatment for stroke, venous interventions, heart valves, hypertension, heart failure and percutenous coronary interventions (PCI).
October 4, 2019 – A new analysis of the PARTNER 3 Trial data found a modest, but significant, improvement in one-year ...
October 4, 2019 – Results of a new economic analysis of the COAPT Trial data found that transcatheter mitral valve ...
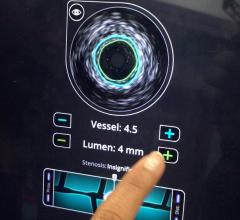
Adjustable parameters on the ACIST® CVI® Contrast Delivery System include, flow rate, volume, pressure limit, rise time ...
October 4, 2019 – RenalGuard Therapy was found to be superior to the POSEIDON method in preventing contrast-induced ...
October 3, 2019 – Five-year results from the PARTNER 2A Trial found patients with severe aortic stenosis (AS) and ...
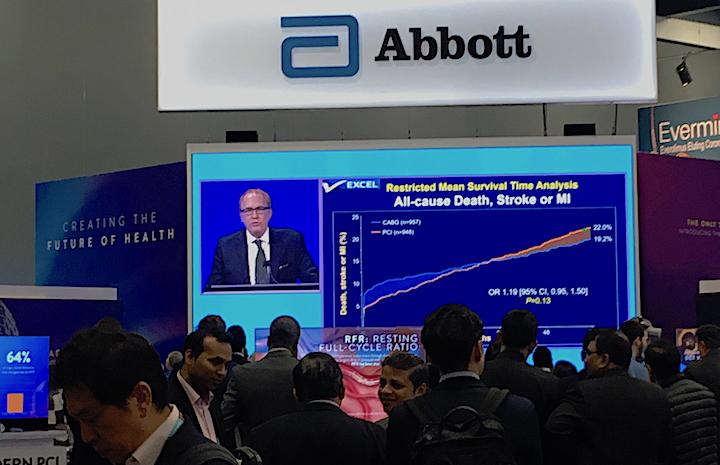
October 3, 2019 – Five-year data from the EXCEL Trial showed patients with left main coronary disease treated with ...
As medical advancements continue to push the boundaries of what is possible in the field of structural heart ...
October 3, 2019 – The three-year results from the COAPT Trial demonstrated that reducing severe secondary mitral ...
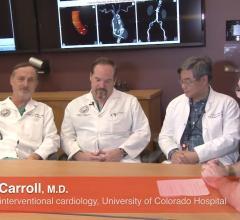
Interview with John Carroll, M.D., director of interventional cardiology, Robert Quaife, M.D., director of advanced ...
October 2, 2019 – The first randomized trial to compare the safety and efficacy of the new Boston Scientific Acurate neo ...
While the current positive revolution in percutaneous coronary intervention (PCI) practice has been made possible by the ...
October 2, 2019 — Democratic presidential candidate Sen. Bernie Sanders (D-VT) was hospitalized with chest pain on Oct ...

Henry Ford Hospital thought leaders regularly speak at the cardiology conferences about new research and technology ...
October 2, 2019 — Philips Healthcare is utilizing Level Ex’s video game app design expertise to train interventional ...
During cardiology fellowship, my institution had a hand manifold system. I found it difficult to use at times, and it ...
 I recently had the opportunity to conduct an onsite visit to the University of Colorado Hospital Heart and Vascular ...
I recently had the opportunity to conduct an onsite visit to the University of Colorado Hospital Heart and Vascular ...
This 360 degree view shows staff at the University of Colorado Heart and Vascular Center performing live transesophageal ...
A discussion with Ruth Fisher, MBA, vice president of the Henry Ford Hospital structural heart program, and Janet Wyman ...


 October 04, 2019
October 04, 2019