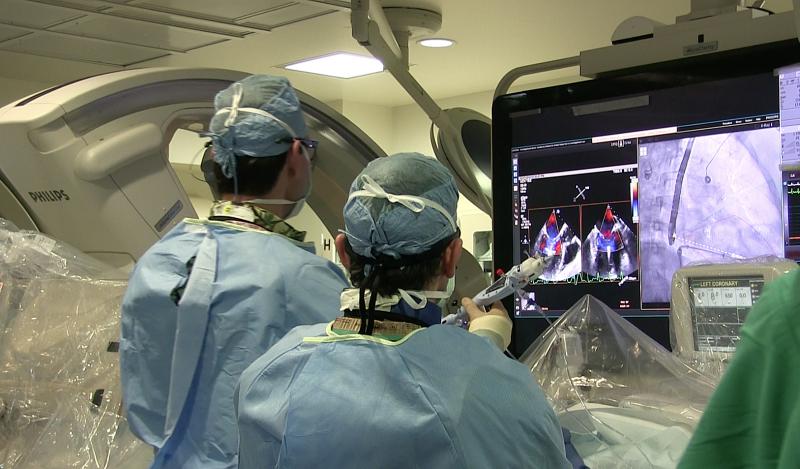
Interview with Carey Kimmelstiel, M.D., FACP, FACC, director, cardiac catheterization laboratory, director ...
Cath Lab
The catheterization lab channel includes content related to interventional technologies for coronary and peripheral artery disease (PAD). Other cath lab transcatheter device technologies covered on this page included percutaneous treatment for stroke, venous interventions, heart valves, hypertension, heart failure and percutenous coronary interventions (PCI).
Navin Kapur, M.D., FAHA, FACC, FSCAI, director, Acute Mechanical Circulatory Support Program and executive director of ...
The key question I am always asked at cardiology conferences is what are the trends and interesting new technologies I ...
Adjustable parameters on the ACIST® CVI® Contrast Delivery System include, flow rate, volume, pressure limit, rise time ...
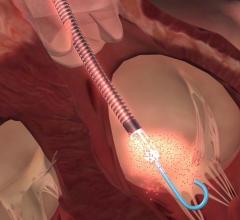
January 22, 2020 – BioVentrix Inc., developer of the first transcatheter device for left ventricular remodeling after a ...
This video shows the first robotic percutaneous coronary interventions (PCI) performed in Germany with the Robocath R ...
January 21, 2020 – Robocath, a company that designs, develops and commercializes cardiovascular robotic systems for the ...
As medical advancements continue to push the boundaries of what is possible in the field of structural heart ...
A venous-venous (VV) extracorporeal membrane oxygenation (ECMO) implantation procedure in a cath lab at Tufts Medical ...
January 15, 2020 — The Society for Cardiovascular Angiography and Interventions (SCAI) released an updated expert ...
January 15, 2020 – Too few people covered by Medicare participated in outpatient cardiac rehabilitation after a heart ...
While the current positive revolution in percutaneous coronary intervention (PCI) practice has been made possible by the ...
January 13, 2020 — The U.S. Food and Drug Administration (FDA) has approved clinical trial that will compare the ...
Haval Chweich, M.D., medical director of the cardiac critical care unit (CCU) at Tufts Medical Center, and assistant ...
Richard Botto, CVT, RCSA, chief cardiovascular technologist, division of cardiology, cardiac cath lab, and Melissa Smith ...
During cardiology fellowship, my institution had a hand manifold system. I found it difficult to use at times, and it ...
January 2, 2020 — In early December 2019, leaders of the European Association for Cardiothoracic Surgery (EACTS) withdre ...
December 30, 2019 — Merit Medical Systems Inc., a leading manufacturer of proprietary devices used primarily in ...


 January 24, 2020
January 24, 2020