Gregg Stone, M.D., presents the results of a pooled analysis of randomized trials of bivalirudin virus heparin in acute ...
Cath Lab
The catheterization lab channel includes content related to interventional technologies for coronary and peripheral artery disease (PAD). Other cath lab transcatheter device technologies covered on this page included percutaneous treatment for stroke, venous interventions, heart valves, hypertension, heart failure and percutenous coronary interventions (PCI).
The late-breaking MitraBridge Study was presented at Transcatheter Cardiovascular Therapeutics (TCT) 2020 meeting showed ...

October 15, 2020 — Shockwave Medical's Intravascular Lithotripsy (IVL) system to treat severely calcified coronary ...
Adjustable parameters on the ACIST® CVI® Contrast Delivery System include, flow rate, volume, pressure limit, rise time ...
This is an example pf the Shockwave Medical Intravascular Lithotripsy (IVL) catheter system designed to break up heavily ...
October 16, 2020 — Positive clinical data from first-in-human studies of the Conformal Medical left atrial appendage ...
October 16, 2020 – Results from the XIENCE 90/28 clinical trials have shown that a shorter course of dual-antiplatelet ...
As medical advancements continue to push the boundaries of what is possible in the field of structural heart ...
October 16, 2020 — The Restore EF Study demonstrates the use of contemporary best practices, including attempting a more ...
October 15, 2020 – The REFLECT II randomized clinical trial evaluating the safety and efficacy of the Keystone Heart ...
October 15, 2020 — The one-year results of the late-breaking DEFINE PCI study showed patients had improved outcomes and ...
While the current positive revolution in percutaneous coronary intervention (PCI) practice has been made possible by the ...
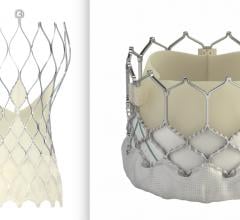
October 15, 2020 – The SCOPE II trial comparing the Boston Scientific Acurate neo vs. Medtronic CoreValve Evolut TAVR ...
October 15, 2020 – The MITHRAS randomized clinical trial found that interventional closure of an iatrogenic atrial ...
October 14, 2020 — American College of Cardiology (ACC) Quality Summit virtual meeting Oct. 8-9, 2020, featured several ...
During cardiology fellowship, my institution had a hand manifold system. I found it difficult to use at times, and it ...
October 14, 2020 — The American College of Cardiology (ACC) and the Cardiovascular Research Foundation (CRF) are ...
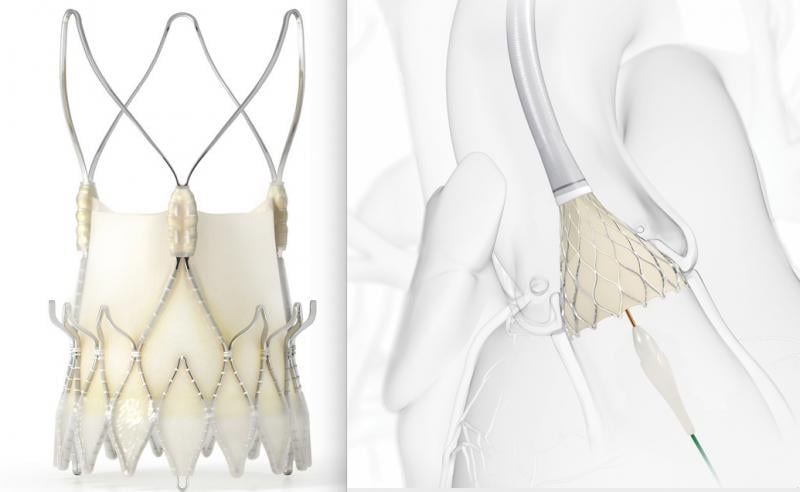
October 14, 2020 — Medtronic announced it is starting a randomized, head-to-head study comparing two transcatheter ...
October 14, 2020 – The first outcomes data from the North American COVID-19 ST-Segment Elevation Myocardial Infarction ...


 October 17, 2020
October 17, 2020