December 23, 2020 — Demonstrations of new technologies and explanations of how they work are among the most popular ...
Cath Lab
The catheterization lab channel includes content related to interventional technologies for coronary and peripheral artery disease (PAD). Other cath lab transcatheter device technologies covered on this page included percutaneous treatment for stroke, venous interventions, heart valves, hypertension, heart failure and percutenous coronary interventions (PCI).

December 23, 2020 — Three clinical trial platforms working together to test the effects of full doses of anticoagulants ...
December 21, 2020 – A proposed ASTM International standard will answer the need for a standardized test method that ...
Adjustable parameters on the ACIST® CVI® Contrast Delivery System include, flow rate, volume, pressure limit, rise time ...
December 16, 2020 — Efemoral Medical announced the first-in-human (FIH) use of the its Efemoral bioresorbable vascular ...
This is an example of the Medis Medical Imaging Quantitative Flow Ratio (QFR) system that offers a fractional flow ...
This is an example of the Siemens Corindus CorPath Cath lab robotic system being used for a percutaneous coronary ...
As medical advancements continue to push the boundaries of what is possible in the field of structural heart ...
December 16, 2020 – Acist Medical Systems Inc. announced a formal distribution partnership with Medis Medical Imaging to ...
December 9, 2020 — SoniVie, an Israeli company developing the Therapeutic Intra-Vascular Ultrasound (TIVUS) System to ...
December 3, 2020 — GE Healthcare is introducing a new version of its robotic driven angiography system for image guided ...
While the current positive revolution in percutaneous coronary intervention (PCI) practice has been made possible by the ...
December 1, 2020 — Here is the list of the most popular content on the Diagnostic and Interventional Cardiology (DAIC) ...
December 1, 2020 — A recent publication demonstrated procedural efficiency for MitraClip transcatheter mitral valve ...
November 24, 2020 — One-year results off the PIONEER III study comparing the safety and efficacy of the Supreme HT ...
During cardiology fellowship, my institution had a hand manifold system. I found it difficult to use at times, and it ...

November 17, 2020 — Since the approval of the first transcatheter aortic valve replacement (TAVR) device in 2011, more ...

November 17, 2020 — Boston Scientific Corp. announced today it is immediately retiring the entire Lotus Edge ...
November 17, 2020 — Diagnostic imaging techniques were able to find the underlying cause of heart attack in many women ...

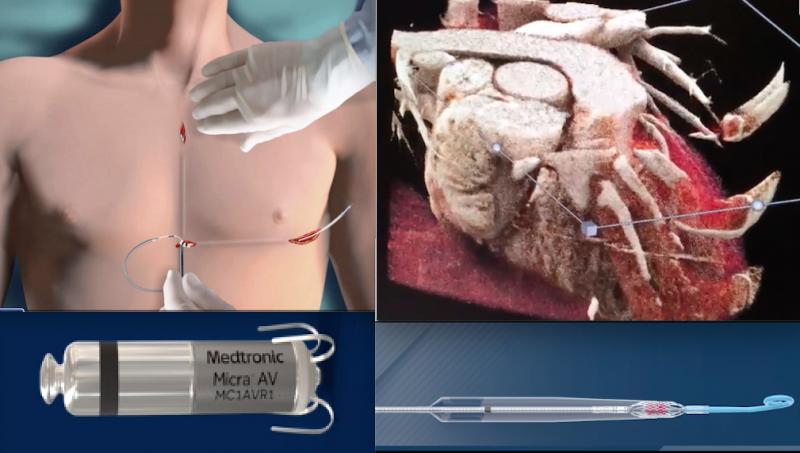
 December 23, 2020
December 23, 2020