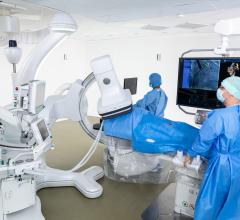
This is an example of the Siemens Corindus CorPath Cath lab robotic system being used for a percutaneous coronary ...
Cath Lab
The catheterization lab channel includes content related to interventional technologies for coronary and peripheral artery disease (PAD). Other cath lab transcatheter device technologies covered on this page included percutaneous treatment for stroke, venous interventions, heart valves, hypertension, heart failure and percutenous coronary interventions (PCI).
December 16, 2020 – Acist Medical Systems Inc. announced a formal distribution partnership with Medis Medical Imaging to ...
December 9, 2020 — SoniVie, an Israeli company developing the Therapeutic Intra-Vascular Ultrasound (TIVUS) System to ...
Adjustable parameters on the ACIST® CVI® Contrast Delivery System include, flow rate, volume, pressure limit, rise time ...
December 3, 2020 — GE Healthcare is introducing a new version of its robotic driven angiography system for image guided ...
December 1, 2020 — Here is the list of the most popular content on the Diagnostic and Interventional Cardiology (DAIC) ...
December 1, 2020 — A recent publication demonstrated procedural efficiency for MitraClip transcatheter mitral valve ...
As medical advancements continue to push the boundaries of what is possible in the field of structural heart ...
November 24, 2020 — One-year results off the PIONEER III study comparing the safety and efficacy of the Supreme HT ...

November 17, 2020 — Since the approval of the first transcatheter aortic valve replacement (TAVR) device in 2011, more ...

November 17, 2020 — Boston Scientific Corp. announced today it is immediately retiring the entire Lotus Edge ...
While the current positive revolution in percutaneous coronary intervention (PCI) practice has been made possible by the ...
November 17, 2020 — Diagnostic imaging techniques were able to find the underlying cause of heart attack in many women ...
November 17, 2020 — A daily dose of omega-3 fatty acids did not reduce the risk of cardiac events, including secondary ...
November 16, 2020 — The use of the more potent antiplatelet medication ticagrelor (Brilinta) was not superior to ...
During cardiology fellowship, my institution had a hand manifold system. I found it difficult to use at times, and it ...
November 13, 2020 — The new U.S. Food and Drug Administration (FDA) cleared XO Score system was successfully used to ...
November 12, 2020 — Abiomed recently announced new PROTECT III study data that demonstrates reduced rates of MACCE ...
Keith Ellis, M.D., is the director of cardiovascular services and the director of the Chest Pain Center at Houston ...


 December 16, 2020
December 16, 2020