January, 27, 2021 — The U.S. Food and Drug Administration (FDA) has granted Occlutech a Breakthrough Device designation ...
Cath Lab
The catheterization lab channel includes content related to interventional technologies for coronary and peripheral artery disease (PAD). Other cath lab transcatheter device technologies covered on this page included percutaneous treatment for stroke, venous interventions, heart valves, hypertension, heart failure and percutenous coronary interventions (PCI).
January 26, 2021 — Pedra Technology, a privately held company, announced today that the U.S. Food and Drug ...
January 26, 2021 — The U.S. Food and Drug Administration (FDA) has cleared Boston Scientific's Synergy Megatron Drug ...
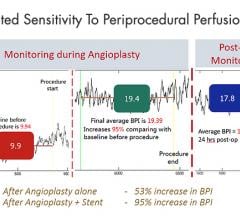
Adjustable parameters on the ACIST® CVI® Contrast Delivery System include, flow rate, volume, pressure limit, rise time ...

With increasing complexity of interventional structural heart disease and congenital heart disease interventions, 3-D ...
January 20, 2021 — The U.S. Centers for Medicare and Medicaid Services (CMS) revised its National Coverage Determination ...

January 19, 2021 – With the postponement of non-essential elective surgeries and medical procedures in 2020 to conserve ...
As medical advancements continue to push the boundaries of what is possible in the field of structural heart ...
January 11, 2021 — Medtronic has received a new expanded indication from Health Canada for its Evolut Transcatheter ...
January 8, 2021 — The U.S. Food and Drug Administration (FDA) has granted 510(k) clearance for the Inari Medical Inc ...
January 6, 2021 — The U.S. Food and Drug Administration (FDA) has granted the designation of breakthrough device to P+F ...
While the current positive revolution in percutaneous coronary intervention (PCI) practice has been made possible by the ...
December 29, 2020 — Edwards Lifesciences announced Dec. 21 that the first patient was treated in the RESTORE clinical ...
December 29, 2020 — Health Canada has approved the expanded use of the Edwards Lifesciences Sapien 3 and Sapien 3 Ultra ...

Here are the top 25 best performing articles on the Diagnostic and Interventional Cardiology (DAIC) website from 2020 ...
During cardiology fellowship, my institution had a hand manifold system. I found it difficult to use at times, and it ...
Here are the top performing 25 videos on the DAIC website in 2020. The picks are based on Google Analytics of the DAIC ...
December 23, 2020 — Demonstrations of new technologies and explanations of how they work are among the most popular ...

December 23, 2020 — Three clinical trial platforms working together to test the effects of full doses of anticoagulants ...

 January 27, 2021
January 27, 2021