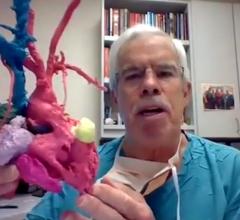
June 7, 2021 — Edwards Lifesciences recently announced that clinical results from the company's transcatheter mitral and ...
Cath Lab
The catheterization lab channel includes content related to interventional technologies for coronary and peripheral artery disease (PAD). Other cath lab transcatheter device technologies covered on this page included percutaneous treatment for stroke, venous interventions, heart valves, hypertension, heart failure and percutenous coronary interventions (PCI).
June 7, 2021 – Cardiologists at Henry Ford Hospital are first in the U.S. and second in the world to implant a ...

June 2, 2021 — The startup company HLT is a part of the Bracco Group, which is developing a new transcatheter aortic ...
Adjustable parameters on the ACIST® CVI® Contrast Delivery System include, flow rate, volume, pressure limit, rise time ...

June 1, 2021 — Here is the list of the most popular content on the Diagnostic and Interventional Cardiology (DAIC) magaz ...
Tom Jones, M.D., director, cardiac catheterization laboratories, Seattle Children’s Hospital, explains some of the new ...

May 26, 2021 — Data presented at hotline and late-breaking trial sessions at the EuroPCR 2021 congress for the updated ...
As medical advancements continue to push the boundaries of what is possible in the field of structural heart ...
May 20, 2021 — Boston Scientific Corporation announced it has initiated the AGENT IDE trial for the Agent Drug-Coated ...
Ehtisham Mahmud, M.D., division chief of cardiovascular medicine, director of interventional cardiology and the cardiac ...
Ehtisham Mahmud, M.D., division chief of cardiovascular medicine, director of interventional cardiology and the cardiac ...
While the current positive revolution in percutaneous coronary intervention (PCI) practice has been made possible by the ...
May 17, 2021 — In patients receiving therapeutic hypothermia after suffering out-of-hospital cardiac arrest, those who ...
May 17, 2021 — The anticoagulant rivaroxaban (Xarelto), in addition to low-dose aspirin, significantly reduced the ...
May 17, 2021 — In patients who received a coronary stent for an ST-elevation myocardial infarction (STEMI) heart attack ...
During cardiology fellowship, my institution had a hand manifold system. I found it difficult to use at times, and it ...

May 16, 2021 — Two months after undergoing renal denervation (RDN) with the ReCor Paradise renal denervation system ...

May 15, 2021 - Transcatheter left atrial appendage occlusion (LAAO) with a Boston Scientific Watchman device was ...
May 15, 2021 — Patients with an elevated risk of stroke due to heart rhythm problems, or atrial fibrillation (AFib) ...

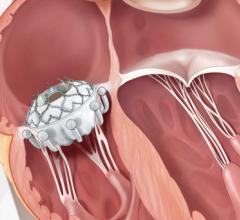
 June 07, 2021
June 07, 2021