October 31, 2008 - Surface modification and drug delivery technologies company SurModics Inc. said today it is providing ...
Cath Lab
The catheterization lab channel includes content related to interventional technologies for coronary and peripheral artery disease (PAD). Other cath lab transcatheter device technologies covered on this page included percutaneous treatment for stroke, venous interventions, heart valves, hypertension, heart failure and percutenous coronary interventions (PCI).
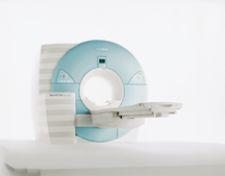
"Potential” can be a loaded word, especially in healthcare. Many clinicians have tagged coronary MR angiography ...
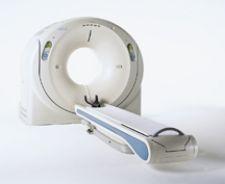
Going into 2009, proponents of coronary CT angiography (CTA) are confident that the scan’s ever-increasing ...
Adjustable parameters on the ACIST® CVI® Contrast Delivery System include, flow rate, volume, pressure limit, rise time ...
Boston Scientific's TAXUS Express2 Atom paclitaxel-eluting coronary stent system is a drug-eluting stent (DES) ...
October 29, 2008 - Nearly 18 months of data from Cappella’s Sideguard I and II self-expanding side branch heart stent in ...
October 30, 2008 - Abbott this week received a 2008 Chicago Innovation Award for its XIENCE V Everolimus Eluting ...
As medical advancements continue to push the boundaries of what is possible in the field of structural heart ...
October 30, 2008 - C. R. Bard Inc. today received approval from the FDA to market the Flair Endovascular Stent ...

There is skepticism among cardiologists whether flat panel detectors can help lower the X-ray dose more than using ...
October 30, 2008 - CHF Solutions said seven studies supporting ultrafiltration therapy’s effectiveness and benefits in ...
While the current positive revolution in percutaneous coronary intervention (PCI) practice has been made possible by the ...
October 27, 2008 – Last week the FDA approved the Endologix Inc. IntuiTrak Delivery System used for the minimally ...
October 27, 2008 - Medtronic Inc. last week announced the U.S. market launch of the Talent Thoracic Stent Graft on the ...
October 27, 2008 - Merit Medical Systems Inc., said last week it received 510(K) clearance from the FDA for the Prelude ...
During cardiology fellowship, my institution had a hand manifold system. I found it difficult to use at times, and it ...
October 27, 2008 - Boston Scientific Corp. last week said the FDA approved its Carotid WALLSTENT Monorail ...
October 22, 2008 - Cook Medical today launched its Advance 18LP Balloon Dilatation Catheter, which has a low crossing ...
October 21, 2008 - MIV Therapeutics Inc., a developer of coatings and drug-delivery systems for cardiovascular stents ...

 October 30, 2008
October 30, 2008


