May 10, 2010 – Patients treated with the Zilver PTX drug-eluting peripheral stent maintained clinical improvement ...
Cath Lab
The catheterization lab channel includes content related to interventional technologies for coronary and peripheral artery disease (PAD). Other cath lab transcatheter device technologies covered on this page included percutaneous treatment for stroke, venous interventions, heart valves, hypertension, heart failure and percutenous coronary interventions (PCI).
May 10, 2010 - The Centers for Medicare and Medicaid Services (CMS) just released a technical correction to the ...

Intracardiac echocardiography (ICE) offers a clearer view of transcatheter devices and electrophysiology (EP) ...
Adjustable parameters on the ACIST® CVI® Contrast Delivery System include, flow rate, volume, pressure limit, rise time ...
May 7, 2010 – Findings from the SPIRIT IV trial, one of the largest randomized clinical trials comparing two drug ...
May 6, 2010 – Patients who can postpone noncardiac surgery for at least six weeks after receiving a coronary ...
May 6, 2010 – After entered the Japanese drug-eluting stent market earlier this year, Abbott's Xience V and ...
As medical advancements continue to push the boundaries of what is possible in the field of structural heart ...
May 5, 2010 – A federal jury awarded more than $29.4 million in damages in a patent infringement lawsuit between ...
May 5, 2010 – Catheter ablation of ventricular tachycardia (VT) is one of the fastest growing electrophysiology ...
May 5, 2010 – A new high-definition intravascular imaging system gained U.S. Food and Drug Administration (FDA) ...
While the current positive revolution in percutaneous coronary intervention (PCI) practice has been made possible by the ...
May 4, 2010 – The U.S. District Court in New Hampshire is being asked this week to review and reverse a jury ...
May 3, 2010 – Excellent safety and clinical outcomes were shown in patients ages 70 and older who received a ...

Several paclitaxel drug-eluting balloons (DEBs) are currently available on the European market, and several others are ...
During cardiology fellowship, my institution had a hand manifold system. I found it difficult to use at times, and it ...
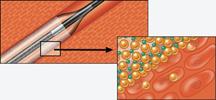
Drug-eluting balloons (DEBs) may offer new options in treating peripheral vessels and restenosis. DEBs already ...

In recent years, many medical device companies saw the underserved lower extremity as an opportunity to enter the ...
April 28, 2010 – Deviating from its focus on intravascular imaging systems and fractional flow reserve (FFR) ...


 May 10, 2010
May 10, 2010









