Here is what you and your colleagues found to be most interesting in the field of cardiology during the month of June ...
Cath Lab
The catheterization lab channel includes content related to interventional technologies for coronary and peripheral artery disease (PAD). Other cath lab transcatheter device technologies covered on this page included percutaneous treatment for stroke, venous interventions, heart valves, hypertension, heart failure and percutenous coronary interventions (PCI).
July 1, 2022 — Despite overall improvements to care for a heart attack, women are less likely to receive timely ...
July 1, 2022 — The carotid artery in 81-year-old Reynold Zamora’s neck was nearly completely blocked with plaque ...
Adjustable parameters on the ACIST® CVI® Contrast Delivery System include, flow rate, volume, pressure limit, rise time ...
June 27, 2022 — Three articles and an accompanying editorial provide information on the effects of Long COVID in the Deu ...
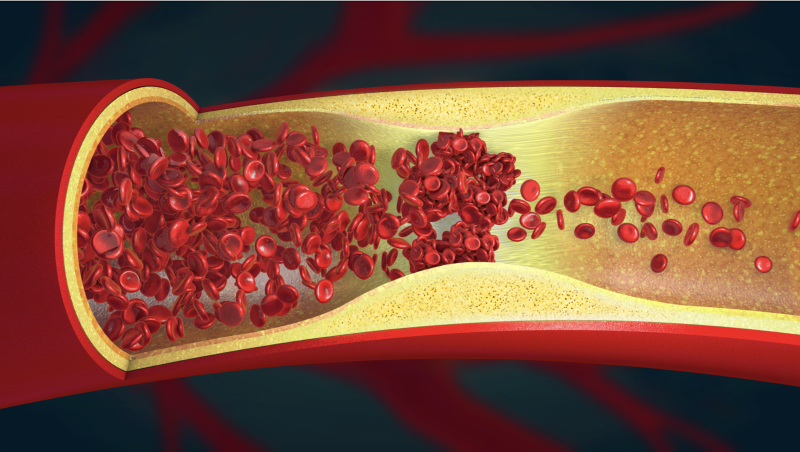
The global thrombectomy devices market is poised to experience substantial expansion, owing to the emergence of ...
June 23, 2022 — Despite overall improvements to care for a heart attack, women are less likely to receive timely ...
As medical advancements continue to push the boundaries of what is possible in the field of structural heart ...
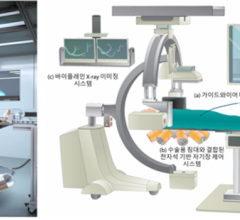
June 17, 2022 — A multidisciplinary team of robotics and electronic systems engineers working with cardiologists and ...
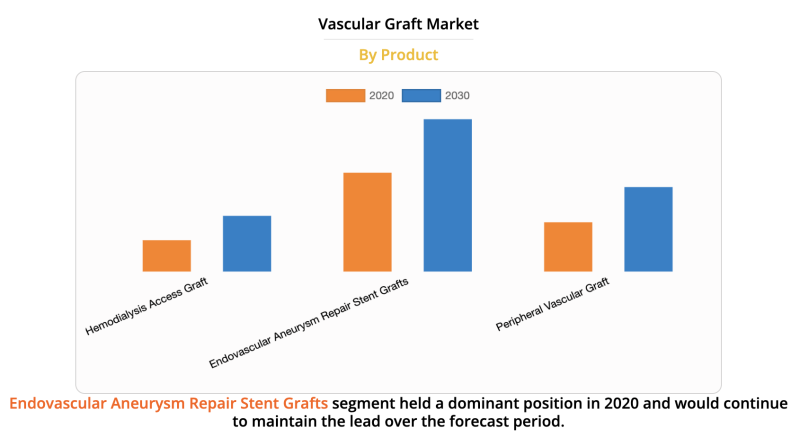
The global vascular graft market size was valued at $4,993.64 million in 2020, and is projected to reach $8,138.68 ...
June 14, 2022 — The Society for Vascular Surgery Vascular Quality Initiative (SVS VQI), a nonprofit organization ...
While the current positive revolution in percutaneous coronary intervention (PCI) practice has been made possible by the ...
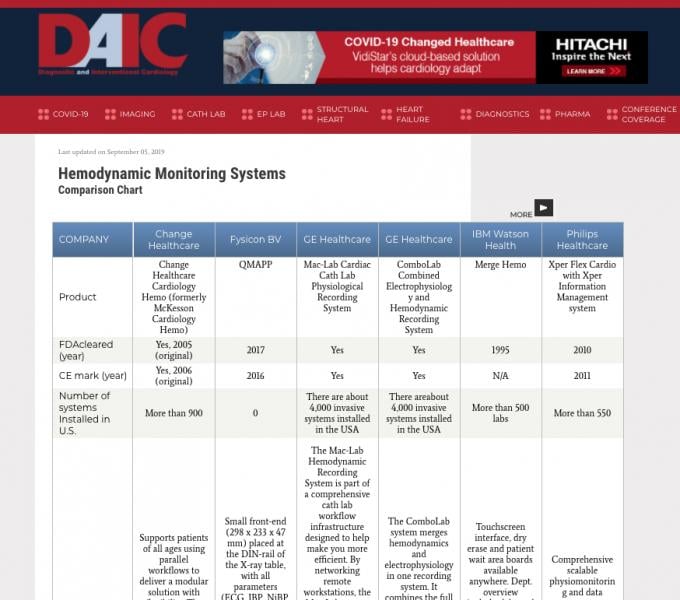
Diagnostic and Interventional Cardiology (DAIC) maintains more than 50 comparison charts of product specifications from ...
According to a new report from Allied Market Research, the global catheters market was valued at $22.7 billion in 2021 ...
June 9, 2022 — Abbott announced late-breaking data for MitraClip, the world's first transcatheter edge-to-edge repair ...
During cardiology fellowship, my institution had a hand manifold system. I found it difficult to use at times, and it ...
June 8, 2022 — The first patient has been enrolled in the FDA IDE BTK (Below-the-Knee) SELUTION4BTK clinical trial ...
June 2, 2022 — Elixir Medical, a developer of innovative, drug-eluting cardiovascular devices, announced print ...
June 2, 2022 — With over 30 years' experience in vascular assessment, Huntleigh, a member of the Arjo family, has ...


 July 05, 2022
July 05, 2022