July 20, 2011 — IDEV Technologies Inc. announced the completion of enrollment in the SUPERB trial, a U.S. Food and Drug ...
Cath Lab
The catheterization lab channel includes content related to interventional technologies for coronary and peripheral artery disease (PAD). Other cath lab transcatheter device technologies covered on this page included percutaneous treatment for stroke, venous interventions, heart valves, hypertension, heart failure and percutenous coronary interventions (PCI).
July 20, 2011 — Ziehm Imaging reports a huge increase in demand for its training programs. In 2010 alone, the number of ...
July 18, 2011 — NSVascular Inc., a newly formed subsidiary of NeuroSigma Inc., has signed an exclusive license with the ...
Adjustable parameters on the ACIST® CVI® Contrast Delivery System include, flow rate, volume, pressure limit, rise time ...
July 18, 2011 — OrbusNeich announced that data from an ex vivo arteriovenous (AV) shunt model of human circulating blood ...
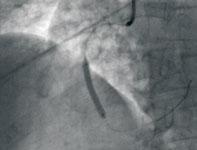
Coronary revascularization does not always lead to coronary reperfusion. When readily available, percutaneous coronary ...
Swedish SCAAR Registry data were presented in May during the EuroPCR meeting in Paris. The 30-day ST-elevated myocardial ...
As medical advancements continue to push the boundaries of what is possible in the field of structural heart ...
Impact of Transradial and Transfemoral Coronary Interventions on Bleeding and Net Adverse Clinical Events in Acute ...

Transradial catheterization is increasingly becoming the access site of choice for many hospitals throughout the United ...
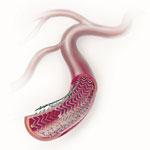
The goal of treating peripheral artery disease (PAD) in the superficial femoral artery (SFA) is limb salvage and ...
While the current positive revolution in percutaneous coronary intervention (PCI) practice has been made possible by the ...

Navigating through the world of coronary stents is about to become more complex. A plethora of new products are on the ...
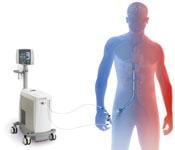
Each year, more than 300,000 people in the United States suffer sudden cardiac death, with some estimates placing this ...
As part of its efforts to expand integration of intravascular ultrasound (IVUS) image guidance with cath lab treatment ...
During cardiology fellowship, my institution had a hand manifold system. I found it difficult to use at times, and it ...
As part of its efforts to expand integration of intravascular ultrasound (IVUS) image guidance with cath lab treatment ...
July 15, 2011 — In a true hybrid suite, both cath lab and open surgical procedures are performed in a single room. In ...
July 14, 2011 — Boston Scientific Corporation has completed patient enrollment in the ASTI post-market clinical follow ...


 July 20, 2011
July 20, 2011






