November 15, 2012 — The U.S. Food and Drug Administration (FDA) granted market clearance for Cook Medical’s Zilver PTX ...
Cath Lab
The catheterization lab channel includes content related to interventional technologies for coronary and peripheral artery disease (PAD). Other cath lab transcatheter device technologies covered on this page included percutaneous treatment for stroke, venous interventions, heart valves, hypertension, heart failure and percutenous coronary interventions (PCI).
Results from the POSEIDON Trial were presented at Transcatheter Cardiovascular Therapeutics (TCT) 2012. Data showed a ...
The PC Trial data presented at TCT 2012 looked at transcather PFO closure vs. medical therapy in preventing cryptogenic ...
Adjustable parameters on the ACIST® CVI® Contrast Delivery System include, flow rate, volume, pressure limit, rise time ...
November 9, 2012 — Boston Scientific Corp. has received approval to update the directions for use (DFU) labeling for ...
November 9, 2012 — Svelte Medical Systems announced it received conditional approval from the U.S. Food and Drug ...
November 9, 2012 — A study found that the use of rosuvastatin prior to angioplasty did not influence the levels of ...
As medical advancements continue to push the boundaries of what is possible in the field of structural heart ...
Toshiba will showcase Spot Fluoro, a dose management tool for Infinix-i vascular X-ray systems. Spot Fluoro allows ...

November 8, 2012 — A clinical trial indicates that using an investigational medical device to close a PFO, or “hole in ...

November 8, 2012 — A hydration regimen tailored to the patient’s fluid status was effective in reducing damage to ...
While the current positive revolution in percutaneous coronary intervention (PCI) practice has been made possible by the ...
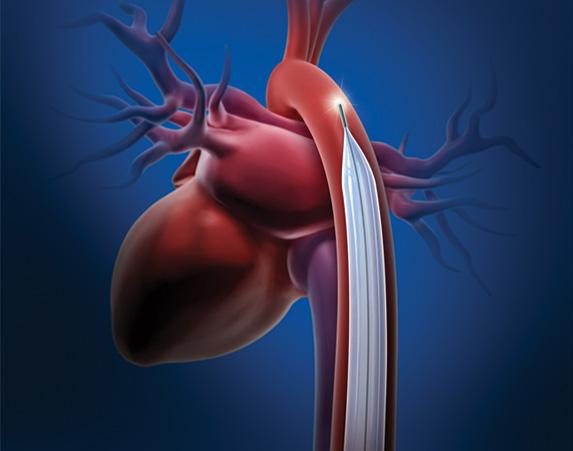
November 8, 2012 — A U.S. Food and Drug Administration (FDA) advisory committee is meeting Dec. 5 to discuss how to ...

November 8, 2012 — Boston Scientific Corp. is extending its reach into the renal denervation market by signing a ...
November 7, 2012 — Kona Medical Inc. announced the initiation of the first clinical study of its device therapy for drug ...
During cardiology fellowship, my institution had a hand manifold system. I found it difficult to use at times, and it ...
November 7, 2012 — Micell Technologies Inc. announced positive data from two clinical studies of its investigational ...
The results of the FAME II Trial were presented at the 2012 Transcatheter Cardiovascular Therapeutics (TCT) meeting. The ...
November 7, 2012 — Athera Biotechnologies AB announced that results from a new study were presented at the Pacific ...

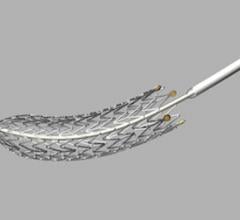
 November 15, 2012
November 15, 2012








