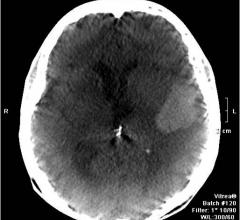
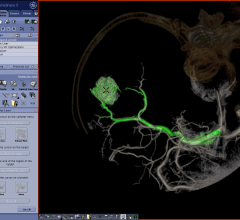
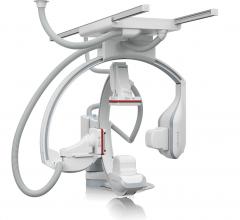
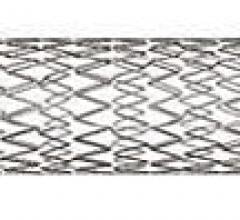
December 6, 2012 — Daiichi Sankyo Inc. and Eli Lilly and Co. announced results of two retrospective, observational ...
Cath Lab
The catheterization lab channel includes content related to interventional technologies for coronary and peripheral artery disease (PAD). Other cath lab transcatheter device technologies covered on this page included percutaneous treatment for stroke, venous interventions, heart valves, hypertension, heart failure and percutenous coronary interventions (PCI).
December 6, 2012 — Giving clinicians a more complete picture while improving safety during interventional procedures ...
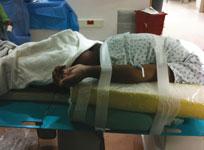
Radial access has been a standard for most patient cases for several years at both the University of Illinois at Chicago ...
Adjustable parameters on the ACIST® CVI® Contrast Delivery System include, flow rate, volume, pressure limit, rise time ...
December 4, 2012 — GE Healthcare announced at RSNA 2012 receipt of U.S. Food and Drug Administration (FDA) clearance for ...

November 30, 2012 — Results of a study investigating the effects of smoking on the antiplatelet medications clopidogrel ...
November 30, 2012 — A large observational study found the increased risk associated with dual antiplatelet therapy cessa ...
As medical advancements continue to push the boundaries of what is possible in the field of structural heart ...
November 30, 2012 — St. Jude Medical Inc. announced that the company’s EnligHTN renal denervation system provides a safe ...
November 26, 2012 — At the 98th annual meeting of the Radiological Society of North America (RSNA) in Chicago, Siemens ...
November 21, 2012 — NDS Surgical Imaging (NDSsi) has expanded its family of advanced LED backlight surgical displays ...
While the current positive revolution in percutaneous coronary intervention (PCI) practice has been made possible by the ...
November 20, 2012 — The environment surrounding a medical imaging system can now be monitored with Toshiba America ...
Mercy Hospital in Chicago developed an interventional program around its hybrid cath labs, fostering collaboration ...
Mercy Hospital in Chicago has developed a successful hybrid cath lab program where various specialties work together for ...
During cardiology fellowship, my institution had a hand manifold system. I found it difficult to use at times, and it ...

November 20, 2012 — The American College of Cardiology (ACC), in collaboration with several other professional societies ...

November 20, 2012 — Ischemic heart disease affects nearly 10 million Americans, and remains the leading cause of death ...
November 20, 2012 — Cordis Corp. announced that the U.S. Food and Drug Administration (FDA) has approved the S.M.A.R.T ...

 December 06, 2012
December 06, 2012