February 26, 2013 — The TIMS Medical division of Foresight Imaging released the TIMS version 3.0 platform to provide ...
Cath Lab
The catheterization lab channel includes content related to interventional technologies for coronary and peripheral artery disease (PAD). Other cath lab transcatheter device technologies covered on this page included percutaneous treatment for stroke, venous interventions, heart valves, hypertension, heart failure and percutenous coronary interventions (PCI).
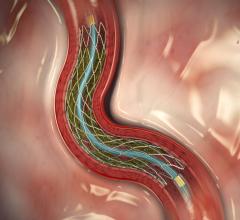
February 25, 2013 — The U.S. Food and Drug Administration (FDA) has approved the 34 and 38 mm lengths of the Medtronic ...
February 22, 2013 — Physicians have new tools for treating coronary chronic total occlusions (CTOs) after Abbott ...
Adjustable parameters on the ACIST® CVI® Contrast Delivery System include, flow rate, volume, pressure limit, rise time ...

February 21, 2013 — Today, Time Magazine releases the dramatic findings of a months-long investigation by contributor ...
February 19, 2013 — CardioLogical Solutions is an emerging cardiovascular device company formed by the merger of ...

February 18, 2013 — Boston Scientific received CE mark approval for the Promus Premier everolimus-eluting platinum ...
As medical advancements continue to push the boundaries of what is possible in the field of structural heart ...
February 13, 2013 — The Society of Interventional Radiology (SIR), an international organization of doctors, scientists ...

February 13, 2013 — St. Jude Medical Inc. announced enrollment of the first patient in the EnligHTN II trial. This post ...
February 13, 2013 — St. Jude Medical Inc announced the first patient implant of its 25 mm Portico Transcatheter Aortic ...
While the current positive revolution in percutaneous coronary intervention (PCI) practice has been made possible by the ...
February 13, 2013 — St. Jude Medical has issued a Class I recall for its Amplatzer TorqVue FX Delivery System used in ...
February 11, 2013 — Silicon Valley Medical Instruments Inc. (SVMI) was granted 510(k) market clearance from the U.S ...
February 11, 2013 — Vascular Nanotransfer Technologies (VNT) says it has developed the industry’s most versatile drug ...
During cardiology fellowship, my institution had a hand manifold system. I found it difficult to use at times, and it ...
February 11, 2013 — The U.S. Department of Veterans Affairs (VA) has selected HP Enterprise Services and subcontractor ...
February 8, 2013 — Identive Group Inc. announced new radio frequency identification (RFID) labels for the medical ...
February 7, 2013 — With more than 100 patients enrolled, Cook Medical has exceeded 50 percent enrollment in the ...


 February 26, 2013
February 26, 2013