March 27, 2014 — Scientists at CBSET, a not-for-profit preclinical research institute dedicated to biomedical research ...
Cath Lab
The catheterization lab channel includes content related to interventional technologies for coronary and peripheral artery disease (PAD). Other cath lab transcatheter device technologies covered on this page included percutaneous treatment for stroke, venous interventions, heart valves, hypertension, heart failure and percutenous coronary interventions (PCI).

March 26, 2014 — Inspire MD Inc. has enrolled the first patient into the CARENET (CARotid Embolic protection study using ...
Adjustable parameters on the ACIST® CVI® Contrast Delivery System include, flow rate, volume, pressure limit, rise time ...
By Dave Fornell, DAIC editor
Data from several cutting-edge device clinical trials will be presented as late-breakers ...
March 21, 2014 — AccessClosure plans to launch the Mynx Ace Vascular Closure Device at the 2014 American College of ...
Today, PinnacleHealth is home to one of the foremost transcatheter aortic valve replacement (TAVR) programs in the world ...
As medical advancements continue to push the boundaries of what is possible in the field of structural heart ...
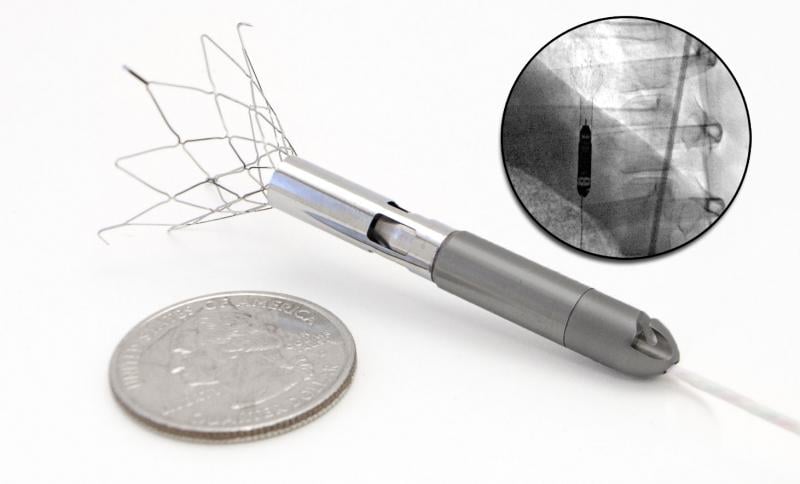
Percutaneous ventricular assist (pVAD) devices offer more hemodynamic support than the 40-year-old gold standard of intr ...
I have watched a trend in medical technology grow from a small ripple a couple years ago into what I expect will be a ...
By Dave Fornell, editor
I have watched a trend in medical technology grow from a small ripple a couple years ago into ...
While the current positive revolution in percutaneous coronary intervention (PCI) practice has been made possible by the ...
March 18, 2014 — Patients with coronary artery disease who received an Endeavor or Resolute drug-eluting stent from ...
March 18, 2014 — The increasing number of percutaneous coronary interventions (PCIs) being performed at low-volume ...
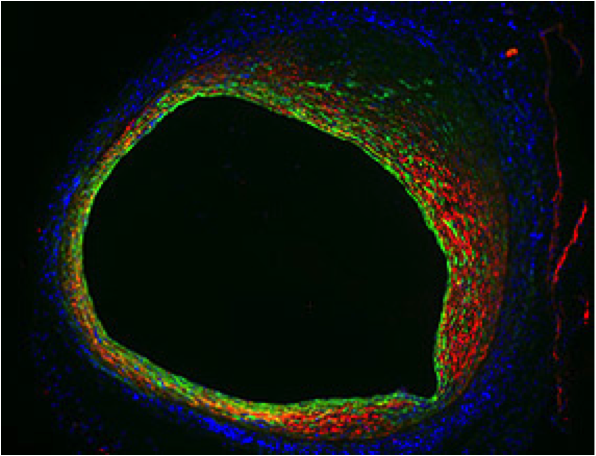
March 13, 2014 — National Institutes of Health researchers have identified a biological pathway that contributes to the ...
During cardiology fellowship, my institution had a hand manifold system. I found it difficult to use at times, and it ...
March 13, 2014 — Boston Scientific Corp. received CE marking for the Rebel Platinum Chromium Coronary Stent System, the ...
March 12, 2014 — Micell Technologies Inc. announced that imaging and clinical results from the DESSOLVE I and DESSOLVE ...
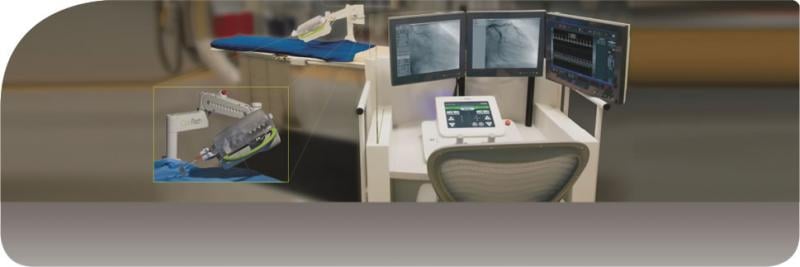
March 12, 2014 — For patients living in rural areas, life-saving procedures such as an angioplasty may only be available ...


 March 27, 2014
March 27, 2014