A big fly in the ointment for widespread adoption of many new technologies is cost. In today’s cost-conscience ...
Cath Lab
The catheterization lab channel includes content related to interventional technologies for coronary and peripheral artery disease (PAD). Other cath lab transcatheter device technologies covered on this page included percutaneous treatment for stroke, venous interventions, heart valves, hypertension, heart failure and percutenous coronary interventions (PCI).
A big fly in the ointment for widespread adoption of many new technologies is cost. In today’s cost-conscience ...
Mr. KH is a 58-year-old male with a past medical history of hypertension and tobacco abuse who presented to the ...
Adjustable parameters on the ACIST® CVI® Contrast Delivery System include, flow rate, volume, pressure limit, rise time ...
June 7, 2014 — Corindus Vascular Robotics has been named the recipient of the 2014 North American New Product Innovation ...
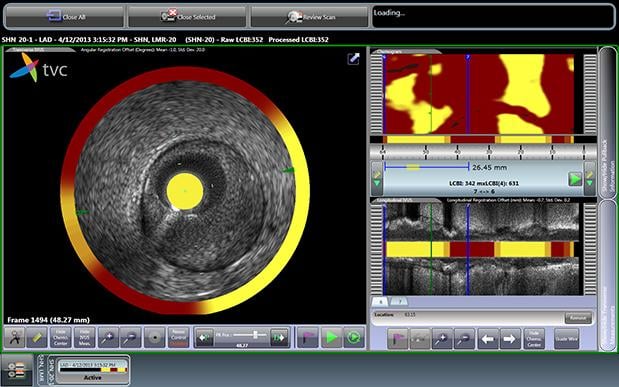
July 3, 2014 — Infraredx Inc. announced the first patient enrolled in PROSPECT II, a multi-center, prospective clinical ...
July 3, 2014 — Mount Sinai Heart at Icahn School of Medicine at Mount Sinai has created an innovative Center for Medical ...
As medical advancements continue to push the boundaries of what is possible in the field of structural heart ...
June 24, 2014 — OrbusNeich announced the completion of enrollment in the prospective, multicenter, all-comers REMEDEE ...

Interventional thought leaders at the American College of Cardiology (ACC) 2014 meeting shared their predictions about ...
While the current positive revolution in percutaneous coronary intervention (PCI) practice has been made possible by the ...
June 16, 2014 — C. R. Bard Inc. announced that the U.S. Food and Drug Administration’s (FDA) Circulatory System Devices ...
June 16, 2014 — New appropriate use expert consensus documents developed by the Society for Cardiovascular Angiography ...

During cardiology fellowship, my institution had a hand manifold system. I found it difficult to use at times, and it ...
June 13, 2014 — Cardinal Health announced the 2 millionth shipment of the Mynx Vascular Closure Device (VCD). The Mynx ...

June 12, 2014 — Three-dimensional imaging known as 3-D quantitative coronary angiography (3D-QCA) accurately identifies ...
June 12, 2014 — Cardiovascular Systems Inc. (CSI) announced that the first patient has been enrolled in its Coronary ...

 July 09, 2014
July 09, 2014







