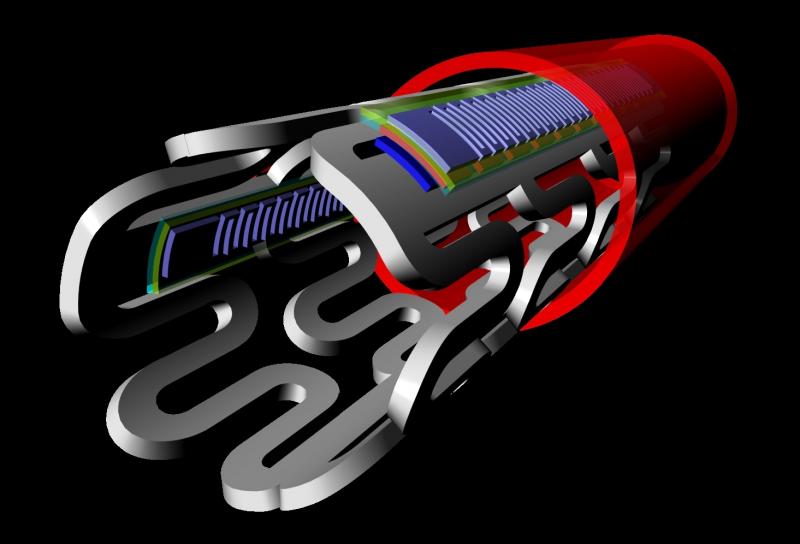
With the recent innovation of flexible microelectronic sensor circuits that can be made from bioresorbable materials, it ...
Cath Lab
The catheterization lab channel includes content related to interventional technologies for coronary and peripheral artery disease (PAD). Other cath lab transcatheter device technologies covered on this page included percutaneous treatment for stroke, venous interventions, heart valves, hypertension, heart failure and percutenous coronary interventions (PCI).
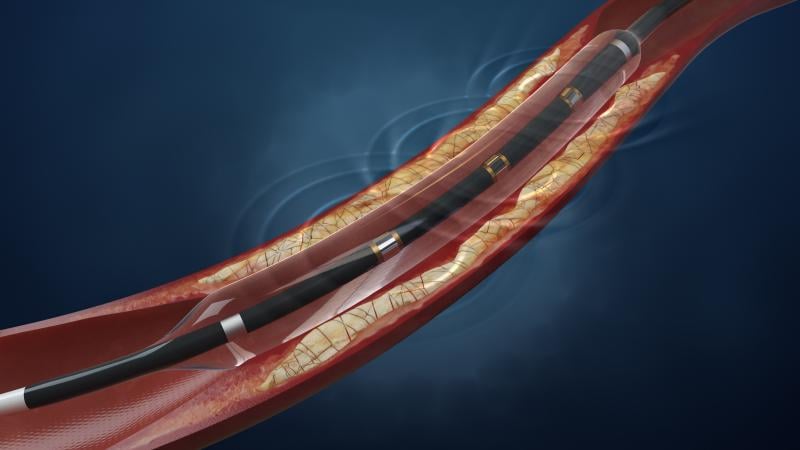
May 28, 2015 — OrbusNeich has announced that the first U.S. patient has been enrolled in the HARMONEE (Harmonized ...
May 27, 2015 — Shockwave Medical announced $40 million in funding, co-led by returning investor Sofinnova Partners and ...
Adjustable parameters on the ACIST® CVI® Contrast Delivery System include, flow rate, volume, pressure limit, rise time ...
May 27, 2015 — A new study evaluating the Boston Scientific Lotus Valve System demonstrated an extremely low rate of ...

May 26, 2015 — Privately-held company Arterial Remodeling Technologies (ART) announced CE Mark clearance for its next ...
May 26, 2015 — St. Jude Medical Inc. announced preliminary results from the ILUMIEN I trial and final results from the ...
As medical advancements continue to push the boundaries of what is possible in the field of structural heart ...
May 22, 2015 — Significantly fewer renal and cardiac events are associated with angioplasty procedures using isosmolar ...
May 22, 2015 — Boston Scientific reported positive, long-term data from the EVOLVE Trial of the Synergy everolimus ...
May 22, 2015 — New clinical data from two different studies show the IN.PACT Admiral drug-coated balloon from Medtronic ...
While the current positive revolution in percutaneous coronary intervention (PCI) practice has been made possible by the ...
May 22, 2015 — St. Jude Medical Inc. announced new results from two clinical studies further supporting the use of its f ...
May 18, 2015 — Amaranth Medical announced plans to report clinical results from the company's ongoing trial of its ...

May 20, 2015 — Adding the HeartFlow FFRCT Analysis to a standard coronary computed tomography angiogram (cCTA) may ...
During cardiology fellowship, my institution had a hand manifold system. I found it difficult to use at times, and it ...
May 19, 2015 — Shimadzu Medical Systems USA announced the first U.S. West Coast installation of its Trinias C-12 high ...

May 19, 2015 — Edwards Lifesciences announced it has temporarily halted its clinical trial of the Fortis mitral transcat ...
May 19, 2015 — Abbott announced that it has received CE Mark for the latest advancement of its Absorb stent system ...

 May 28, 2015
May 28, 2015