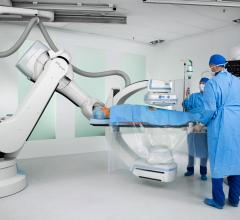
Angiography alone for procedural image guidance in the catheterization lab is sometimes not enough, and it is helpful to ...
Cath Lab
The catheterization lab channel includes content related to interventional technologies for coronary and peripheral artery disease (PAD). Other cath lab transcatheter device technologies covered on this page included percutaneous treatment for stroke, venous interventions, heart valves, hypertension, heart failure and percutenous coronary interventions (PCI).
Key to ongoing U.S. healthcare reform are efforts to reduce costs by eliminating unnecessary medical procedures and ...

July 1, 2015 - CardiAQ Valve Technologies (CardiAQ) announced that its second-generation transcatheter bioprosthetic ...
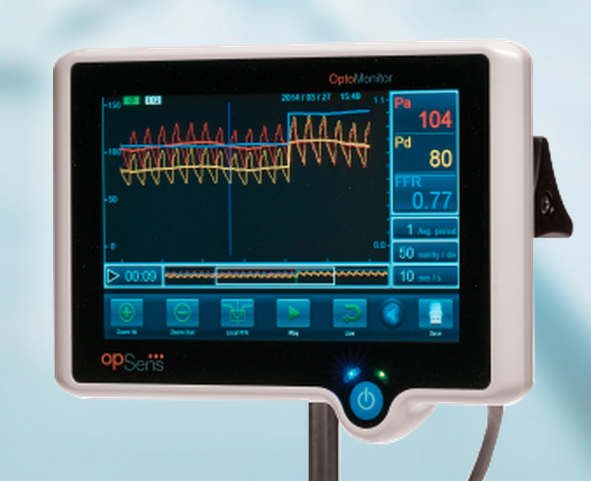
Adjustable parameters on the ACIST® CVI® Contrast Delivery System include, flow rate, volume, pressure limit, rise time ...

June 30, 2015 - Cardiac Dimensions announced the first patients have been enrolled in the REDUCE FMR clinical trial ...

June 30, 2015 - The American College of Cardiology (ACC), Heart Rhythm Society (HRS) and Society for Cardiovascular ...
June 30, 2015 - Merge Healthcare Inc. announced that it has entered into a strategic partnership with Toshiba America ...
As medical advancements continue to push the boundaries of what is possible in the field of structural heart ...
June 26, 2015 - Canadian hospital William Osler Health System (Osler), based in Ontario, has awarded Siemens Healthcare ...
June 26, 2015 - Cheetah Medical announced that it is now offering the U.S. Food and Drug Administration (FDA)-cleared ...
Interview at the American Society of Echocardiography (ASE) annual meeting with Federico Asch, M.D., M.D., FACC, FASE ...
While the current positive revolution in percutaneous coronary intervention (PCI) practice has been made possible by the ...
Interview with Steve Goldstein, M.D., director, noninvasive lab, Medstar Washington Heart Institute, Washington, D.C ...
June 23, 2015 - InspireMD Inc. announced that its CGuard embolic prevention system reported positive results in the PARA ...
June 23, 2015 - Medtronic plc announced the U.S. Food and Drug Administration (FDA) approval and U.S. launch of the ...
During cardiology fellowship, my institution had a hand manifold system. I found it difficult to use at times, and it ...
June 22, 2015 - The Medicines Company announced the approval of cangrelor (Kengreal) by the U.S. Food and Drug ...
June 19, 2015 - Edwards Lifesciences Corp. announced U.S. Food and Drug Administration (FDA) approval of the Edwards ...
June 18, 2015 - Stentys announced in April that it received CE Marking for its new Self-Apposing stent system ahead of ...

 July 02, 2015
July 02, 2015