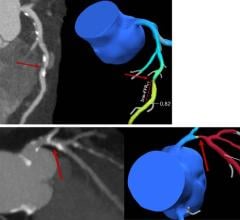
October 30, 2023 — Abbott announced late-breaking data from the LIFE-BTK clinical trial evaluating the Esprit BTK ...
Cath Lab
The catheterization lab channel includes content related to interventional technologies for coronary and peripheral artery disease (PAD). Other cath lab transcatheter device technologies covered on this page included percutaneous treatment for stroke, venous interventions, heart valves, hypertension, heart failure and percutenous coronary interventions (PCI).
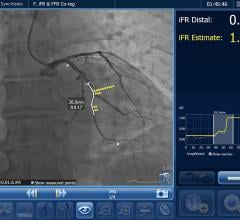
October 26, 2023 — Royal Philips, a global leader in health technology, announced the latest results demonstrating the ...
October 25, 2023 — Shockwave Medical, Inc., a pioneer in the development and commercialization of transformational ...
Adjustable parameters on the ACIST® CVI® Contrast Delivery System include, flow rate, volume, pressure limit, rise time ...
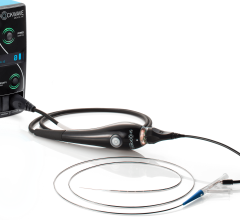
October 25, 2023 — Information on a new double lumen catheter was presented by Nabil Dib, MD, MSc, FACC, FESC, during ...
October 20, 2023 — Over the coming days, Philips will be presenting its latest solutions in cardiology and new late ...
As medical advancements continue to push the boundaries of what is possible in the field of structural heart ...
October 19, 2023 — Edwards Lifesciences Corporation announced the company's EVOQUE tricuspid valve replacement system ...
October 16, 2023 — A door-to-balloon (D2B) time of 90-minutes or less is associated with improved outcomes for heart ...
October 16, 2023 — GE HealthCare (Nasdaq: GEHC) announced US FDA 510(k) clearance of Allia IGS Pulse - the latest ...
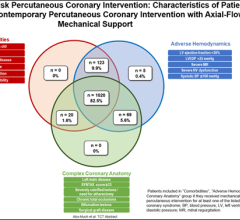
While the current positive revolution in percutaneous coronary intervention (PCI) practice has been made possible by the ...
October 16, 2023 — Shimadzu Medical Systems USA, a subsidiary of Shimadzu Corporation, announced the first U.S ...
October 10, 2023 — Elixir Medical, a developer of innovative cardiovascular technologies, announced it will present ...
September 27, 2023 — Boston Scientific announced the FDA clearance of the AVVIGOTM+ Multi-Modality Guidance System, a ...
During cardiology fellowship, my institution had a hand manifold system. I found it difficult to use at times, and it ...
September 19, 2023 — An advanced CT test can identify individuals with stable angina at a reduced risk of three-year ...
September 18, 2023 — Biosense Webster, Inc., a global leader in cardiac arrhythmia treatment and part of Johnson & ...
September 14, 2023 — BIOTRONIK announced the two-year-results from the BIOLUX P-III BENELUX all-comers registry ...


 October 30, 2023
October 30, 2023