Medtronic announced Feb. 3 that the U.S. Food and Drug Administration (FDA) revised the CoreValve’s instructions for use ...
Cath Lab
The catheterization lab channel includes content related to interventional technologies for coronary and peripheral artery disease (PAD). Other cath lab transcatheter device technologies covered on this page included percutaneous treatment for stroke, venous interventions, heart valves, hypertension, heart failure and percutenous coronary interventions (PCI).
February 2, 2016 — Medtronic plc announced the recent CE Mark and commercial launch for an expanded size matrix of the ...
January 29, 2016 — Intact Vascular Inc. announced that positive six-month results from its Tack Optimized Balloon ...
Adjustable parameters on the ACIST® CVI® Contrast Delivery System include, flow rate, volume, pressure limit, rise time ...
January 29, 2016 — IBM Watson Health announced that the 2015/2016 Best in KLAS: Software & Services report recognized ...
January 26, 2016 — Conavi Medical Inc. (formerly Colibri Technologies Inc.) has received U.S. Food and Drug ...
January 25, 2016 — Now, medical technology purchasers and users have an instant online forum to rate and review technolo ...
As medical advancements continue to push the boundaries of what is possible in the field of structural heart ...
January 25, 2016 — The suspension of the medical device excise tax will have positive consequences for the U.S. medical ...
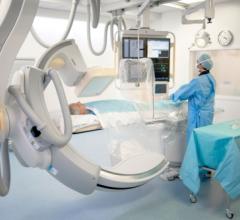
GE Healthcare is taking the next leap in image quality performance, quantification and workflow with the introduction of ...
January 20, 2016 — On the 100th anniversary of the Endurance expedition to Antarctica led by Sir Ernest Shackleton ...
While the current positive revolution in percutaneous coronary intervention (PCI) practice has been made possible by the ...
At the beginning of each year, I always try to determine what the next big cardiovascular technology advances to watch ...
The U.S. Food and Drug Administration (FDA) Circulatory System Devices Panel is set to review data and offer ...
January 18, 2016 — Vivasure Medical announced Conformité Européenne (CE) Mark approval of the world’s first fully ...
During cardiology fellowship, my institution had a hand manifold system. I found it difficult to use at times, and it ...
January 18, 2016 — Medtronic plc announced that the IN.PACT Admiral drug eluting balloon (DEB) has received CE ...
January 15, 2016 — Teleflex Inc. has acquired privately held Nostix LLC, developer of innovative tip confirmation ...
January 15, 2016 — Patients between the ages of 40 and 70 who undergo aortic valve replacement (AVR) may fare better ...


 February 03, 2016
February 03, 2016