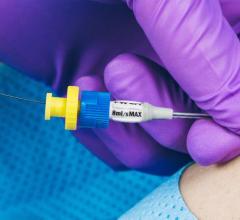
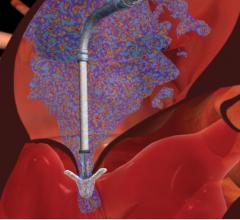
August 10, 2016 — Mercator MedSystems Inc. announced the enrollment of the first critical limb ischemia (CLI) patients ...
Cath Lab
The catheterization lab channel includes content related to interventional technologies for coronary and peripheral artery disease (PAD). Other cath lab transcatheter device technologies covered on this page included percutaneous treatment for stroke, venous interventions, heart valves, hypertension, heart failure and percutenous coronary interventions (PCI).
August 10, 2016 — Access Scientific introduced the PowerWand XL, a companion to the PowerWand All-in-One, in July. The ...

In the past few years, concern has skyrocketed from interventional cardiologists and cath lab staff over radiation dose ...
Adjustable parameters on the ACIST® CVI® Contrast Delivery System include, flow rate, volume, pressure limit, rise time ...
August 8, 2016 — Updated recommendations from the American Academy of Neurology (AAN) states that catheter-based closure ...
August 5, 2016 — The Centers for Medicare and Medicaid Services (CMS) has reassigned MitraClip transcatheter mitral ...
August 5, 2016 — Osprey Medical Inc. said it received commitments from investors for approximately $28 million ...
As medical advancements continue to push the boundaries of what is possible in the field of structural heart ...

August 5, 2016 — Here are the top 20 most popular current content on the Diagnostic and Interventional Cardiology ...

August 4, 2016 — Medtronic announced CE (Conformité Européenne) mark market clearnace for the self-expanding ...
As more procedures move into the cath lab, growing complexity is fueling an increase in procedural volume and the need ...
While the current positive revolution in percutaneous coronary intervention (PCI) practice has been made possible by the ...
August 3, 2016 — The U.S. Food and Drug Administration (FDA) has granted market clearance to Rex Medical’s bioresorbable ...
August 2, 2016 —The U.S. Food and Drug Administration (FDA) granted market clearance for W. L. Gore & Associates’ Gore ...

Patients with atrial fibrillation (AF or Afib) are high risk for stroke due to the formation of thrombus emboli in the ...
During cardiology fellowship, my institution had a hand manifold system. I found it difficult to use at times, and it ...
July 27, 2016 — Penumbra Inc. announced U.S. commercial availability of its latest thrombectomy device, the ACE68 ...
July 27, 2016 — Edwards Lifesciences Corp. announced that Health Canada has approved the Edwards Sapien 3 transcatheter ...
July 27, 2016 — A number of states mandate public reporting of mortality outcomes following certain cardiac procedures ...


 August 10, 2016
August 10, 2016