December 7, 2016 — At the 102nd Scientific Assembly and Annual Meeting of the Radiological Society of North America ...
Cath Lab
The catheterization lab channel includes content related to interventional technologies for coronary and peripheral artery disease (PAD). Other cath lab transcatheter device technologies covered on this page included percutaneous treatment for stroke, venous interventions, heart valves, hypertension, heart failure and percutenous coronary interventions (PCI).
December 6, 2016 — The Spectranetics Corp. announced in late November that its Stellarex 0.014-inch drug-coated ...

December 5, 2016 — Here is the list of the top 20 most popular pieces of content on the Diagnostic and Interventional ...
Adjustable parameters on the ACIST® CVI® Contrast Delivery System include, flow rate, volume, pressure limit, rise time ...
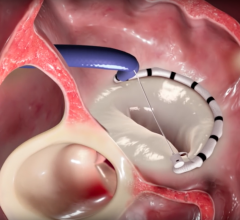
December 2, 2016 — Edwards Lifesciences Corp. announced this week it has agreed to acquire Valtech Cardio Ltd., a ...
November 27, 2016 — At the 2016 Radiological Society of North America (RSNA) annual meeting, Ziehm Imaging presents its ...
Corindus Vascular Robotics received U.S. Food and Drug Administration (FDA) 510(k) clearance for its CorPath GRX, the ...
As medical advancements continue to push the boundaries of what is possible in the field of structural heart ...
November 21, 2016 – Healthcare providers seeking versatile imaging technology for their angiography needs can find a ...
November 21, 2016 – A single-center study sponsored by the National Institutes of Health (NHLBI) failed to show an early ...
November 18, 2016 — InspireMD Inc. announced in mid-October the online publication of positive clinical data in a new ...
While the current positive revolution in percutaneous coronary intervention (PCI) practice has been made possible by the ...
November 18, 2016 — Mitralign Inc. announced the successful compassionate use treatment of a patient suffering from tric ...
DAIC Editor Dave Fornell takes a video tour of some of the most innovative new interventional cardiology technologies he ...
A discussion with Juan Granada, M.D., about transcatheter mitral valve advancements and device challenges at the Transca ...
During cardiology fellowship, my institution had a hand manifold system. I found it difficult to use at times, and it ...
A discussion with guidewire expert Dimitri Karmpaliotis, M.D., Ph.D., FACC, about the basics of interventional guidewire ...
A discussion with Torsten Vahl, M.D., about advancements in transcatheter valve repair technology, including new devices ...
A discussion with Simon Dixon, M.D., MBChB, on the use of fractional flow reserve-computed tomography (FFR-CT) to ...


 December 07, 2016
December 07, 2016