January 11, 2017 — Avinger Inc. recently announced the U.S. launch of an enhanced version of the company’s Lightbox ...
Cath Lab
The catheterization lab channel includes content related to interventional technologies for coronary and peripheral artery disease (PAD). Other cath lab transcatheter device technologies covered on this page included percutaneous treatment for stroke, venous interventions, heart valves, hypertension, heart failure and percutenous coronary interventions (PCI).
January 10, 2017 — BioVentrix Inc. announced in December the first closed-chest Revivent TC TransCatheter Ventricular ...
January 10, 2017 — Medinol announced in December positive twelve-month clinical results from the BIONICS study. The ...
Adjustable parameters on the ACIST® CVI® Contrast Delivery System include, flow rate, volume, pressure limit, rise time ...
January 10, 2017 — Teleflex Inc. announced that its Arrow VPS Rhythm Device with optional TipTracker technology has been ...
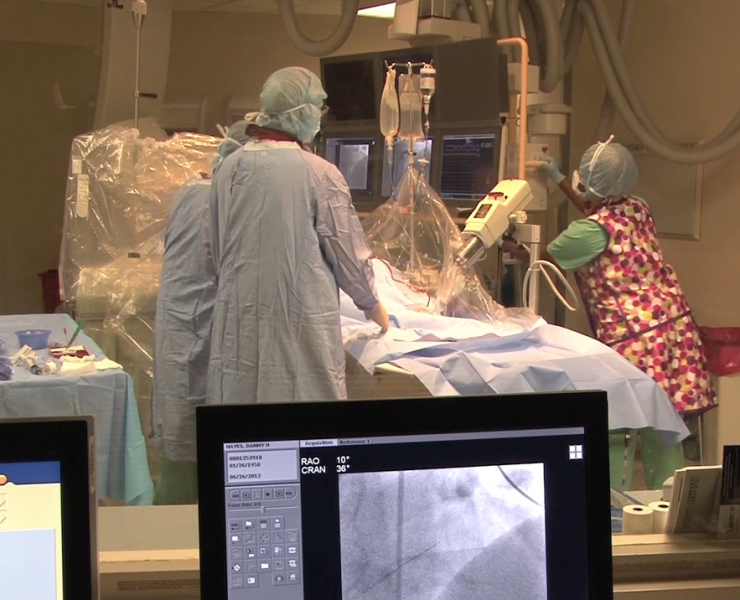
Effective July 1, 2017 for Medicare heart attack patients, the the Centers for Medicare and Medicaid Services (CMS) will ...
January 9, 2017 — BioCardia Inc. announced in December the issuance of United States Patent No. 9,517,199 relating to a ...
As medical advancements continue to push the boundaries of what is possible in the field of structural heart ...
January 9, 2017 — Whale Imaging Inc. announced U.S. Food and Drug Administration (FDA) 510(k) clearance of the G-Arm Duo ...
ITN and DAIC Editor Dave Fornell takes a tour of some of the most innovative new technologies being displayed on the ...
January 5, 2017 — A new report published by Allied Market Research forecasts that the global thrombectomy devices market ...
While the current positive revolution in percutaneous coronary intervention (PCI) practice has been made possible by the ...
January 5, 2017 — Abbott announced it completed the acquisition of St. Jude Medical Inc. The transaction provides Abbott ...
January 4, 2017 — A new minimally invasive technique for repairing the most common cardiac birth defect in extremely ...
January 4, 2015 — At RSNA 2016, Siemens Healthineers unveiled its 510(k)-pending robot-supported Artis pheno angiography ...
During cardiology fellowship, my institution had a hand manifold system. I found it difficult to use at times, and it ...
January 3, 2017 — The Impella CP heart pump (Abiomed) demonstrated no improvement in mortality for patients with ...
This video, provided by Valtech, demonstrates the Cardioband transcatheter mitral annuloplasty system. It allows ...
January 2, 2017 — The U.S. Food and Drug Administration (FDA) hopes to build clinical evidence through new registries ...


 January 11, 2017
January 11, 2017